
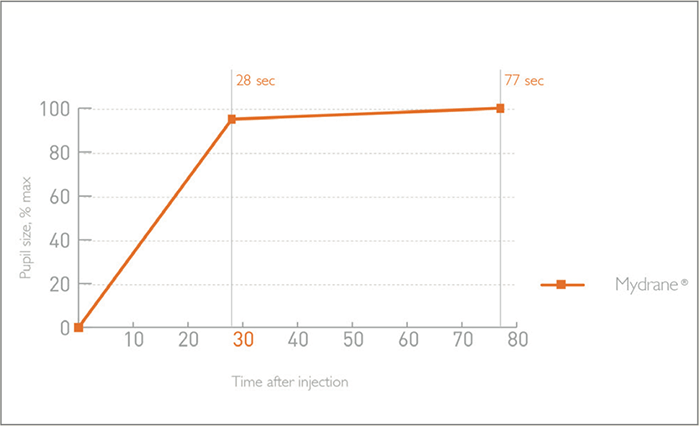
The lengthiest part of a cataract procedure often isn’t the surgery itself – it is the time needed for patients to achieve mydriasis. Dilating eyedrops may be the traditional standard of care, but there are drawbacks associated with delayed mydriasis: longer pre-operative preparation and systemic side effects. Now, there is a faster, more convenient and safer way: Mydrane®. Mydrane® is a standardized, ready-to-use preparation for intracameral mydriasis that contains a unique combination of two mydriatrics (tropicamide 0.02 percent and phenylephrine 0.31 percent) and the anesthetic lidocaine (1 percent). Intracameral injection of 0.2 ml of Mydrane® after the first incision induces rapid and stable mydriasis; 95 percent dilation is achieved within 30 seconds after administration (see Figure 1)(1)(2). Furthermore, the effects are long-lasting: mydriasis is maintained during the whole procedure, unlike eyedrops, where re-mydriasis might be required (3).

In a Phase III trial involving over 555 patients (4), Mydrane® was proven to be a safe and effective alternative to eyedrops for initiating and maintaining large and stable mydriasis and analgesia during cataract surgery. It has also been associated with significantly less patient discomfort during the procedure, and surgeons reported that cataract procedures were easier in cases where Mydrane® was used, as compared with dilating eyedrop use – particularly with lens insertion. Most importantly, less time is needed to complete the surgery with Mydrane®. Thanks to the simplified procedure in which the unique combination is injected directly into the intracameral chamber in the operating room, the time that patients need to wait during the pre-operative stage in the outpatient department is significantly reduced. Not only is the routine use of intracameral mydriatics more comfortable for the patient, it could also modify the organization of surgical sessions, allowing surgeons to take advantage of more flexibility and rapidity for the turnover of patients. Mydrane® is available in Europe as a box of 20 sterile 0.6 mL ampules and 20 sterile filter needles, and is indicated for cataract surgery to obtain mydriasis and intraocular anesthesia during the surgical procedure (2). Mydrane® is the first marketed product for this indication in Europe, offering a ready-to-use standardized solution with regulatory protection as an alternative to the DIY process already used in some countries. 1. Indicated for cataract surgery to obtain mydriasis and intraocular anesthesia during the procedure in adults only. Mydrane is indicated for patients who have demonstrated a satisfactory pupil dilation with topical mydriatic therapy during pre-operative visit.
Mydrane® in Action
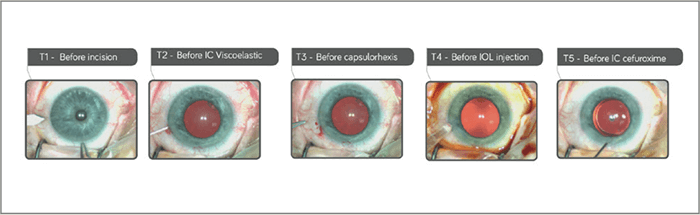
Mydrane® offers the fastest way to obtain an efficient and stable dilation compared with mydriatic eye drops (3). Within 30 seconds of administration, 95 percent of pupil dilation is obtained – and subsequent viscoelastic injection often has an add-on effect. Figure 1 shows three separate cases in which mydriasis has been obtained within 30 seconds after Mydrane® administration, with one case achieving dilation in only 10 seconds. Figure 2 shows the speed of pupil dilation, with 95 percent of pupil dilation obtained with Mydrane® within 30 seconds. Mydrane® induces a long-lasting pupil dilation that remains stable throughout the surgery, allowing IOL implantation under good conditions (Figure 3).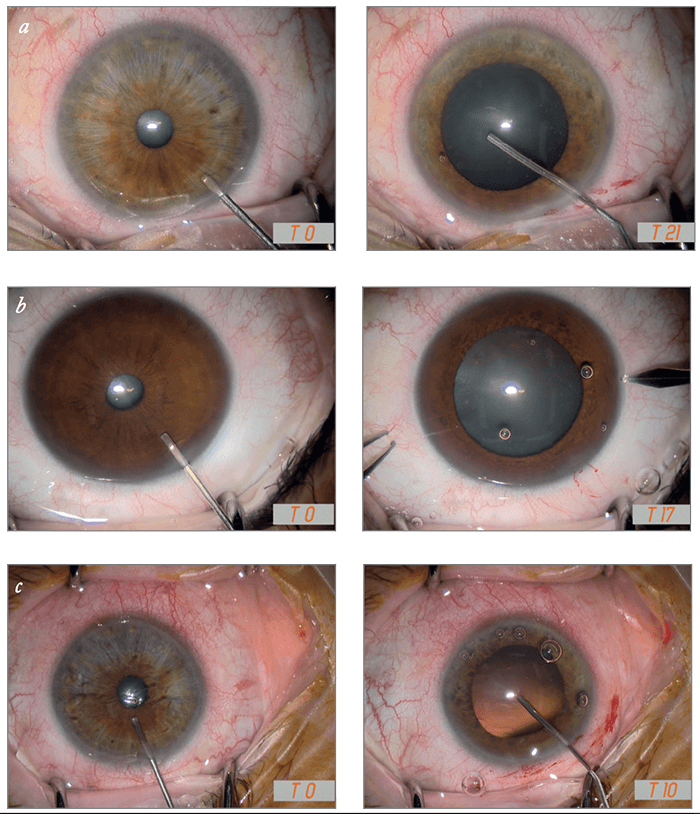
References
- Internal report on phase II clinical study on small pupils. Efficacy and safety assessment of intracameral T2380 (Fixed combination of lidocaine, phenylephrine and tropicamide) to obtain mydriasis in patients with small pupils for cataract surgery by phacoemulsification. Pilot, multicentre, open Study. Data summary in Clinical Overview (LT2380-PII-09/12). Data on file Marketing Authorization dossier. Mydrane Summary of Product Characteristics (September 29, 2017). Laboratoires Théa, Clermont-Ferrand, France. Available at: bit.ly/mydranesmpc F Chiambaretta et al., “Pupil dilation dynamics with an intracameral fixed combination of mydriatics and anesthetic during cataract surgery”, J Cataract Refract Surg, In press. M Labetoulle et al., “Evaluation of the efficacy and safety of a standardized intracameral combination of mydriatics and anaesthetics for cataract surgery”, Br J Ophthalmol, 7, 976–985 (2016). PMID: 26531052.
