- Current glaucoma therapies lower intraocular pressure (IOP), but don’t address the primary cause of raised IOP
- Multiple topical medications for IOP management can be a regimen compliance nightmare
- Micro-invasive glaucoma surgeries (MIGS) directly address outflow resistance and avoid many of the risks associated with traditional surgical treatments like trabeculectomy
- Not only can MIGS devices reduce IOP, but also the number of topical drops needed to control it
Topical hypotensive glaucoma medications have been the cornerstone of glaucoma therapy for over four decades. This is partly because they work – when used exactly as directed, they reduce patients’ intraocular pressure (IOP). But it’s also partly because there wasn’t really an alternative other than trabeculectomy – with all of the issues that can involve. The problems with topical glaucoma medications are well-known: many patients need multiple drops to control their IOP (e.g. a β-blocker and a prostaglandin analog). If patients take one drop after another, the second drop can wash out the first, and multiple, multi-instillation-per day regimens mean that considerable discipline is required to adhere to them. If patients don‘t comply, IOP control goes out the window, with inevitable consequences for their outcomes.
Although we don’t yet completely understand glaucoma’s pathology, it’s clear that IOP is raised in patients with open angle glaucoma because of a failure in the aqueous drainage system. Instead of flowing through Schlemm’s canal and into the appropriate collector channels:, aqueous humor collects, and IOP rises (1). Topical glaucoma medications act either to decrease aqueous production, or increase aqueous outflow.
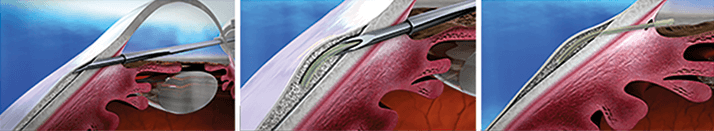
Today, there are new options. Tiny, easily implantable drainage devices that directly address outflow resistance, and at the same time side-step many of the risks and difficulties associated with existing surgical glaucoma treatments: these strategies are collectively referred to as micro-invasive glaucoma surgery (MIGS). One example of a MIGS device is the iStent (Glaukos, Laguna Hills, CA, USA;
Figure 1). Delivered via an ab interno procedure, the surgeon inserts the iStent through a clear corneal incision, advances it through the trabecular meshwork, and implants it into Schlemm’s canal
(Figure 2). The procedure avoids any manipulation of the iris, conjunctiva or sclera, which preserves other surgical and medical options in case future interventions are needed.
The device treats the chief pathology of primary open angle glaucoma by bypassing the source of resistance, and re-establishing a pressure-dependent aqueous pathway for fluid, from the anterior chamber, through the trabecular meshwork to Schlemm’s canal and the collector channels. Research has confirmed the importance of treatments like this, which mine traditional outflow pathways that would otherwise continue to deteriorate (2). Increasing outflow through the intranasal quadrants with this device significantly lowers IOP due to the high presence of collector channels (3) – and the physiological preservation of the trabecular meshwork ensures the maintenance of a natural episcleral back-pressure (of approximately 8–11 mmHg) with a minimal risk of hypotony. Ocular hypotensive medications are stopped postoperatively, and then added back as necessary to lower IOP to the patient’s targeted level.
MIGS a good match for cataract? It’s known that phacoemulsification reduces IOP, and a clinical trial of a single iStent placed immediately after cataract surgery in 58 patients with uncontrolled primary open-angle glaucoma and cataract demonstrated not only significant IOP reductions 12 months after the procedure relative to baseline levels (21.7 mmHg at baseline; 12 months later 17.4 mmHg) but also a reduction in the mean number of glaucoma medications required (1.6 at baseline; 0.4 at 12 months), with half of patients with an IOP ≤18 mmHg being able to discontinue hypotensive medicine after 12 months (4); results confirmed by a second study (5). The most common device-related adverse events in the first trial were stent lumen obstruction (7 eyes) and stent malposition (6 eyes) (4); of the 36 patients enrolled in the second trial, only two stents were malpositioned (5). More recently, the placement of two iStents, two clock hours apart in the eye has been evaluated in two studies, one in Canada (6) and one with the second generation iStent (iStent inject) in Europe (7); the effect on IOP appears to be additive, with the studies producing IOP reductions of 32.4 and 39.7 percent, respectively.
It would appear that placing iStent(s) during the cataract surgery procedure doesn’t impact upon the excellent safety profile of cataract surgery alone, so it seems to me to be a reasonable option to offer an iStent to patients requiring cataract surgery who also have mild to moderate open angle glaucoma and currently take topical hypotensive medications. From the patient’s perspective, there are plenty of reasons to want alternatives to medication alone – the cost of glaucoma medication has risen significantly in recent years (8), proper instillation of eye drops can be difficult for many patients, particularly the elderly, and the hassle and potential side effects of the drops are deterrents to proper regimen adherence. MIGS surgery can potentially avoid all of this – and at a minimum, reduce the number of topical medications required, and with it, the hassle that goes along with them. Improving surgical treatments for glaucoma is important, but so is prevention. Campaigns for earlier detection and intervention of the condition will hopefully reduce the need for riskier forms of surgery. Currently, over 60 million people in the world are estimated to suffer from either open angle or angle closure glaucoma (9). Many of these people are assumed to be unaware of their disease state and may therefore not receive appropriate treatment. The more advanced their glaucoma, the greater the chance that the collector channels – the normal drainage system in the eye – will be damaged by the disease, chronic medication or both. If these patients could be diagnosed and treated earlier, MIGS devices could potentially lower their IOP enough to reduce or even eliminate the need for other treatment – an outcome that would significantly improve their quality of life over many years to come. There’s a reason that MIGS devices are big news nowadays. They decrease IOP in a more controlled fashion than other treatments currently in use and reduce the risk of postoperative complications. Titratable therapy with multiple stents offers the option of reducing pressure to a greater extent when needed. As the implantation procedure is fast and straightforward, with a rapid postoperative recovery period, this could make performing the procedure appealing to both general ophthalmologists and glaucoma surgeons alike. Patients are likely to appreciate the benefits of fewer medications too.
Steven Vold is the Medical Director of Vold Vision, Fayetteville, Arkansas, US, and is a co-founder of the American-European Congress of Ophthalmic Surgery. Vold also co-founded the Silicon Valley-based ophthalmic device company Ocunetics, Inc. whose intellectual property was exclusively licensed by IRIDEX Corporation in 2011.
Other MIGS technologies
Hydrus Microstent (Ivantis Inc.) The world’s first “intracanalicular scaffold,” the Hydrus Microstent is approximately the size of an eyelash and is made from an elastic, biocompatible nickel-titanium alloy. When inserted, it bypasses the trabecular meshwork and dilates Schlemm’s canal to lower IOP. The implant is currently under evaluation in the Hydrus II and Hydrus IV clinical trials.CyPass Micro-Stent (Transcend Medical) A supraciliary stent that creates a controlled outflow pathway to the suprachoroidal space. Openings all along the length of the stent tubing allow aqueous flow. The CyPass Micro-Stent has been clinically available in Europe since 2009, it is currently only available in the United States by participation in clinical trials. Xen Gel Stent (Aquesys, Inc.) Made of a soft, collagen-based gelatin, the Xen is compressible and conforms to the ocular tissue, minimizing issues associated with synthetic materials. It can be implanted either alone or in conjunction with cataract surgery. CE marked, the stent is classified as an investigational device in the United States and is therefore only available under an Investigational Device Exemption (IDE).
InnFocus Microshunt (InnFocus Inc.) Implanted through an ab externo needle track, the Microshunt moves aqueous fluid from the anterior chamber to a subconjunctival or sub-Tenon flap. It is made of a proprietary material called SIBS, previously used in cardiac stents and shown to cause minimal inflammation. The Microshunt received a CE mark in 2012 and is currently undergoing the FDA clinical trial approval process in the United States. iTrack Canaloplasty Microcatheter (Ellex) Canaloplasty aims to increase conventional outflow by catheterizing and dilating Schlemm’s canal. The iTrack microcatheter allows circumferential cannulation, and ophthalmic viscoelastic device injection, and it has an illuminated tip for transscleral visualization during insertion. The iTrack has received both FDA marketing approval and the CE mark.
References
- R. Rosenquist, D. Epstein, S. Melamed, et al.,“Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculectomy”, Curr. Eye Res., 8, 1233–1240 (1989). D.H. Johnson, Y. Matsumoto, “Schlemm’s canal becomes smaller after successful filtration surgery”, Arch. Ophthalmol., 118, 1251–1256 (2000). J. Zhou, G.T. Smedley, “Trabecular bypass: effect of Schlemm canal and collector channel dilation”, J. Glaucoma, 15, 446–455 (2006). D. Spiegel, W. Wetzel, T. Neuhann, et al., “Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery”, Eur. J .Ophthalmol., 19, 393–399 (2009). A.M. Fea, “Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma – Randomized double-masked clinical trial”, J. Cataract Refract. Surg., 36, 407–412 (2010). doi: 10.1016/j.jcrs.2009.10.031. I.I. Ahmed, l.J. Katz, D.F. Chang, et al., “Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma”, J. Cataract Refract. Surg.m 40, 1295–300 (2014). doi: 10.1016/j.jcrs.2014.07.004. L. Voskanyan, J. García-Feijoó, J.I. Belda, et al., “Prospective, unmasked evaluation of the iStent® inject system for open-angle glaucoma: synergy trial”, Adv. Ther. 31, 189–201 (2014). doi: 10.1007/s12325-014-0095-y. N.R. Rylander, S.D. Vold, “Correspondence: Cost Analysis of Glaucoma Medications”, Am. J. Ophthalmol., 145, 1108–1109 (2008) H.A. Quigley, A.T. Broman, “The number of people with glaucoma worldwide in 2010 and 2020”, Br. J. Ophthalmol., 90, 262–267 (2006).
