The use of an autologous sclera patch graft in human ocular surgery (by Swedish ophthalmologist Sven Larsson) goes back to the mid-1940s; at the same time, R. Townley Paton founded the first eye bank in New York. Preserved human sclera from eye bank donor eyes would come to replace the use of autologous sclera, but back then transplant surgeries had to take place no more than 48 hours after the death of the donor.
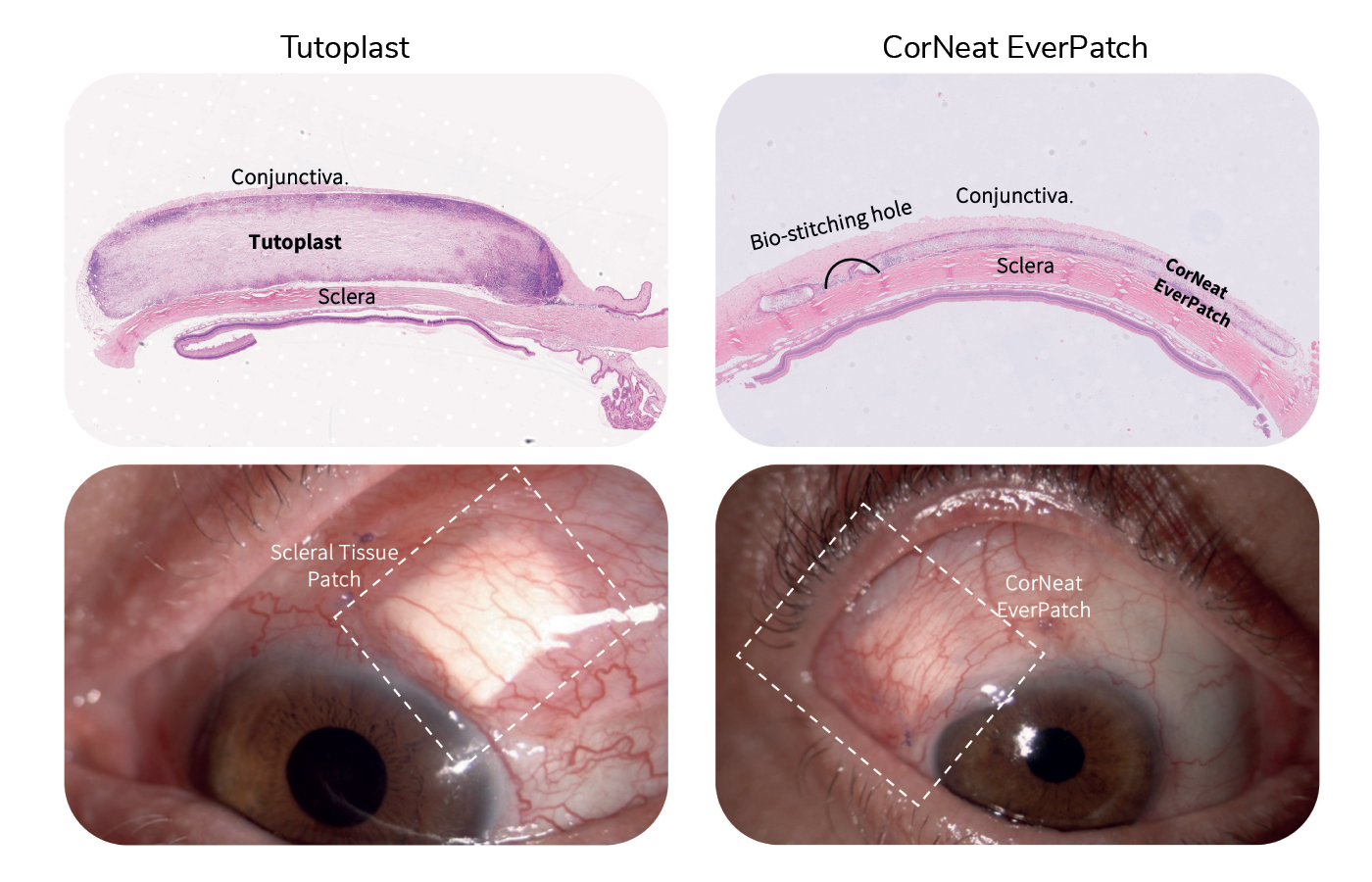
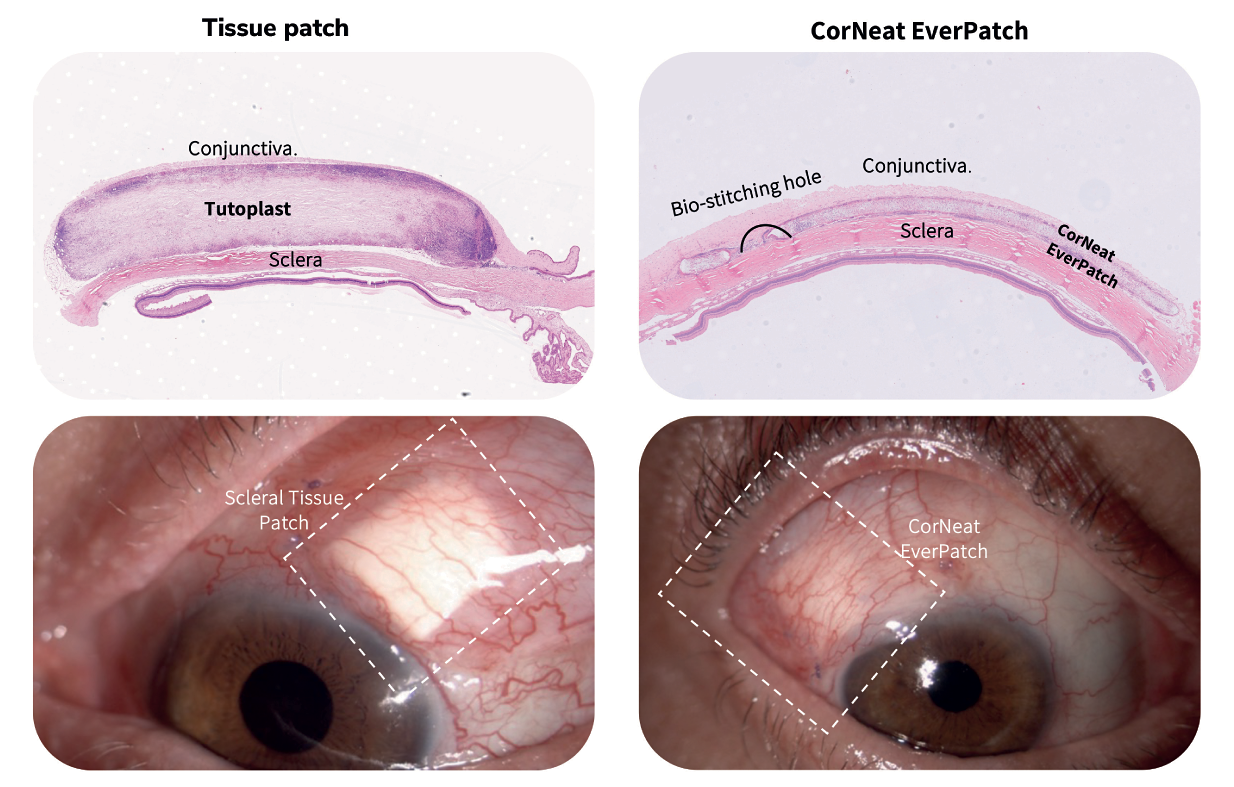
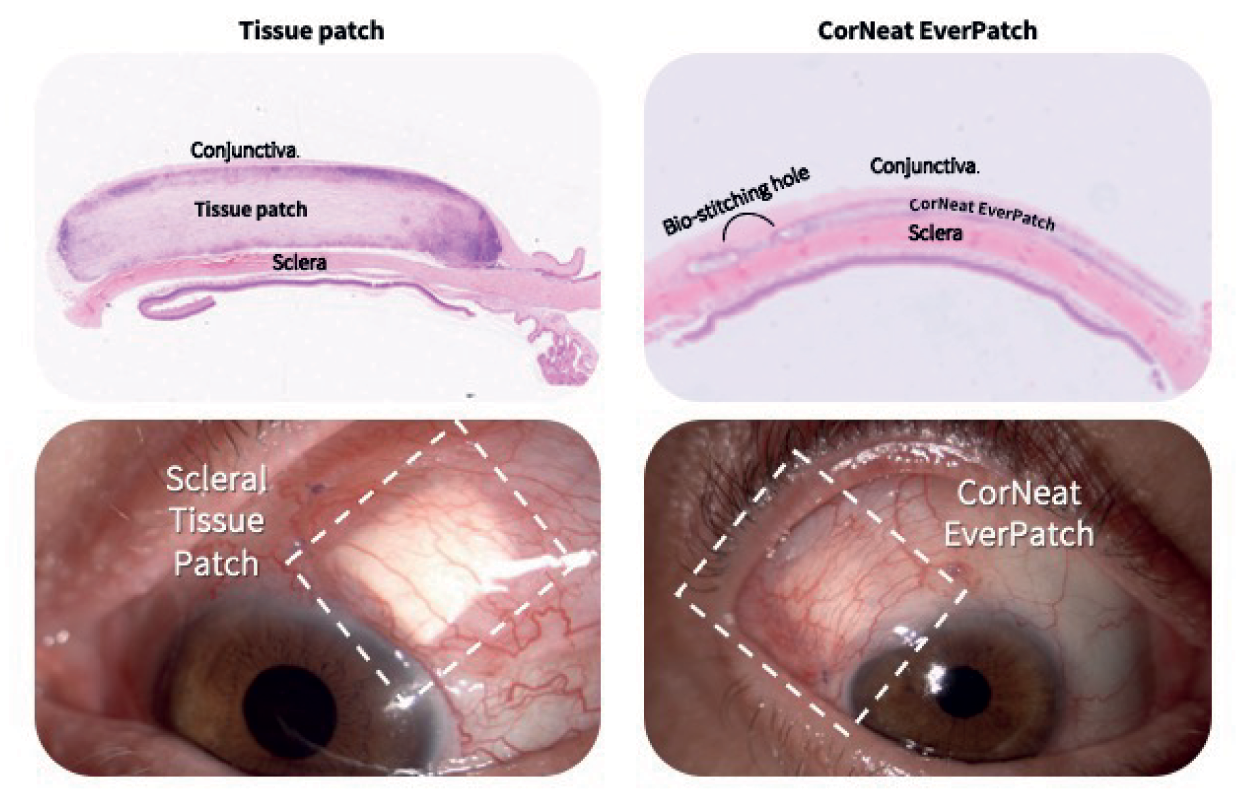
Advancements in techniques to preserve ocular tissues over the following decades meant that sclera grafts could be stored for much longer, and scleral reinforcement has become the gold standard as a safety measure in glaucoma drainage device (GDD) surgeries. The use of human tissue in these surgeries, however, has been beset by disadvantages; for example, it is degraded by the body’s inflammatory cells, and there is a limited amount of graft material available. But thanks to the CorNeat EverPatch, these challenges are now surmountable.
As the first alternative to tissue that is synthetic, non-degradable, and sterile, the CorNeat EverPatch remains in the tissue following GDD surgery, and conceals the glaucoma tube shunt for the patient’s life. “The non-degradability of this medical device will have a dramatic impact on the rate of late complications,” says Dr. Gilad Litvin, CorNeat Vision’s Co-Founder and Chief Medical Officer. “And as it is not harvested from cadaveric origin, it is safer, reducing the risk of infection, specifically disease transmission.”
The design and the features of the CorNeat EverPatch were optimized to address the surgeon’s needs, simplifying the procedure. Where current tissue patches have a thickness of 400-500 μm to allow for protection until native scar tissue forms to conceal the tube, the nondegradable EverPatch is much thinner – just 100 μm – which reduces tension on the surgical wound and makes closing it easier for the surgeon. This “durability, flexibility, and resistance to cheese wiring allows easy and accurate manipulation and suturing,” explains Almog Aley-Raz, CorNeat Vision’s CEO and VP R&D.
The EverPatch is based on the EverMatrix™, the company’s disruptive platform material technology. The EverMatrix™ is biocompatible, nondegradable biomimetic material that imitates the micro-structure of the human Extracellular Matrix (ECM) – the collagen mesh providing the structural and biochemical support to surrounding cells. It can be seen as a natural habitat for human fibroblasts, which play a critical role in wound healing and constitute the most common connective tissue cells. Invivo studies have shown the abundant presence of fibroblasts and collagen fibrils within the EverMatrix™ several weeks following its implantation with no adverse immune system response. In addition to the CorNeat EverPatch, the company develops two additional disruptive implants based on its platform technology, the CorNeat KPro – an artificial cornea intended to restore sight in corneally blind individuals – and the CorNeat eShunt – the first shunt that drains in the intraconal space, prolonging efficacy.
Supported by this innovative technology, the CorNeat EverPatch looks set to revolutionize ophthalmic surgery, allowing for scleral implantation in regions where access to donor tissue is scarce, offering ease of handling and manipulation, and eliminating the risk of tube exposure and disease transmission. Since it was soft launched in the US in December 2023, the CorNeat EverPatch has been implanted in dozens of leading ophthalmic centers, gaining excellent feedback from physicians.
The CorNeat EverPatch is available for evaluation and sales in the US and other countries relying on FDA regulation
