Optical coherence tomography (OCT) instruments have been used in the clinic for over 20 years now, and it’s almost impossible to describe how big an impact this technology made. The ability to visualize the layers of the retina in three dimensions with a rapid and non-invasive procedure has profoundly transformed our understanding of both how retinal pathologies occur and how to treat them. The technology has evolved at an astonishing rate. We started with time-domain (TD) OCT imaging, which gave way to the far superior spectral-domain (SD) OCT, which is now being supplanted by swept-source (SS) OCT. Scan rates have increased – the first commercially available TD OCT instrument scanned at 7,000 A-scans per second; today, there are commercially available SS-OCT instruments that can perform 100,000 A-scans per second. OCT angiography (OCT-A) represents almost as big an advance as the introduction of OCT two decades ago.

The principle behind Swept Source OCT Angiography (SS OCT Angio™) is simple. A vessel is scanned four times in rapid succession, generating four images. An algorithm (OCTARA) takes those four images and performs a subtraction analysis. Everything that moves (i.e. the erythrocytes in the vessels) remains. Everything else disappears, generating the angiograph (see Box 1: OCT-A of the Trevi and the Four Rivers fountains). It is important to realize that SS OCT Angio™ can show areas of non-perfusion (as demonstrated in the case studies presented by Madgy Moussa, Cases 2 & 3), but currently, we cannot see leakage or the far periphery. As Florence Coscas shows (Case 4), SS OCT Angio™ complements fluorescein angiography (FA), indocyanine green angiography (ICG-A) and OCT-B scans at the diagnosis step, and substantially reduces the need for FA at follow up appointments. Nevertheless, the ability to perform angiography, quickly and non-invasively is a huge improvement over the conventional, gold-standard methods of FA or ICG-A. There’s no need to prepare the patient before, or monitor them after the procedure. There is no risk of inducing nausea or allergic reactions (and occasionally, anaphylaxis) from injecting the patient with the contrast agent. OCT-A takes seconds to perform, and can be repeated as often as is necessary.
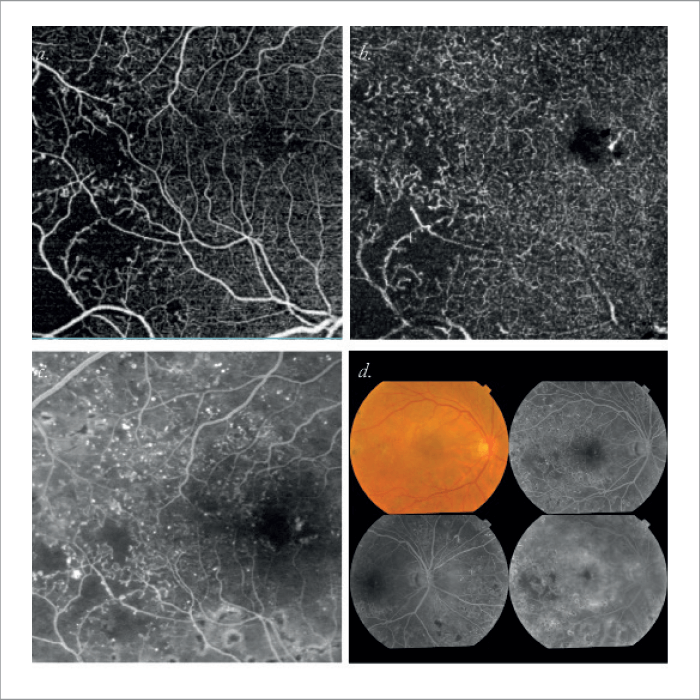
Magdy Moussa, Tanta University, Egypt Case 2. Right eye of female patient aged 56 years with a diagnosis of severe non-proliferative diabetic retinopathy. SS OCT Angio (a,b) is superior to FA (c) and fundus photography (d) in delineating macular ischemia and perifoveal non-perfusion.
However, it’s the combination of swept-source imaging and OCT-A (SS OCT Angio™) that represents the greatest advance to date. Topcon’s SS OCT instruments emit light at 1050 nm; traditional SD OCT instruments all emit light at ~840 nm. There are a number of key advantages of using the longer wavelength: it can penetrate through media opacities like cataract or vitreous hemorrhage, and crucially, penetrates further into the retina – down to the choroidal-scleral interface, with minimal loss of signal strength. This is helping reveal structures like Haller’s layer (Case 1) that were almost impossible using other non-invasive imaging modalities. As we’ll show in the following case examples, SS OCT Angio™ can do a lot more than was first thought; it can help to guide ophthalmologists in cases that would otherwise be ambiguous, even with FA and other multimodal imaging techniques. Finally, it’s important to note that there’s only one commercially-available instrument that can image the choroidal vasculature available today, and that’s the Topcon DRI OCT Triton.
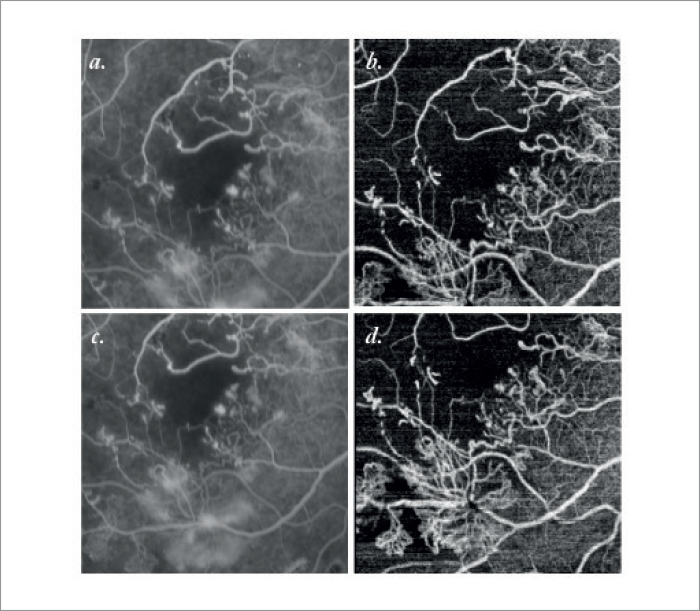
OCT-A is still considered to be an emerging imaging technique, but today it can provide users with clear visualizations of blood flow both in retinal and choroidal tissue. As SS OCT Angio™ can obtain depth-resolved images of retinal and choroidal tissue, it means you can obtain layer-by-layer visualizations of the entirety of a choroidal neovascularization (CNV). As SS OCT Angio™ yields this rapidly and non-invasively, it’s an attractive method for following CNV activity. The questions were: what do we need to look for on the SS OCT-A images to determine the activity and the degree of proliferation of the lesion? How do we assess lesion persistence and recurrence, or conversely, stabilization and healing? Which vessels are mature and which are “quiescent”? Understanding this is vital, in terms of making the right decision when it comes to anti-VEGF agents: to inject, or not to inject? Last year, we evaluated the ability of SD OCT-A versus traditional and multimodal imaging approaches (histopathology, FA, ICG-A and OCT), to determine CNV activity status in a prospective case series of 80 eyes (73 consecutive patients) with exudative AMD (aged 79.4 ± 5.3 years) (1). This revealed five essential OCT-A criteria that are required to identify CNV activity. As illustrated in Case 4, these are:
- Shape A well-defined CNV lesion (tortuous lacy/ wheel-shaped appearance) – in contrast to one with long, filamentous linear vessels.
- Multiple anastomoses and loops Presence of anastomoses and loops, in contrast to absence of anastomoses and loops.
- Branching pattern Numerous tiny capillaries, in contrast to rare, large mature vessels.
- Vessel termini morphology Presence of a peripheral arcade, in contrast to a “dead tree” appearance
- Perilesional, hypo-intense halo Presence of a perilesional hypo-intense halo, considered as regions of choriocapillaris alteration, in contrast to absence of a perilesional hypo-intense halo.
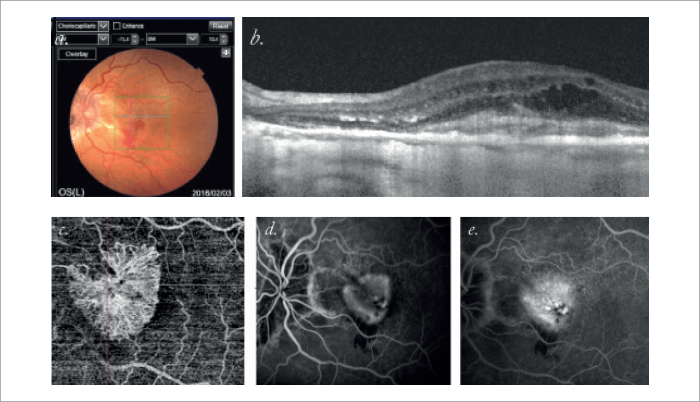
Together, these five criteria allow highly specific and sensitive description of the most relevant morphologic features of a neovascular lesion. In a new study, we found that SS OCT Angio™ together with the OCT-B scans, gave the same response as conventional FA with OCT-B scans, in over 90 percent of cases in clarifying lesion status (two experienced observers). The fact that SS OCT Angio™ can today identify specific features of a neovascular network is impressive, and important as this can be a valuable tool to support diagnosis and treatment decisions. Changes in vascular pattern may be a direct sign of how a CNV varies over time and how it responds to the therapy itself, and this can be rapidly and non-invasively achieved with SS OCT Angio™. This article is a summary of the Topcon-sponsored satellite symposium held at Euretina, Copenhagen, September 2016. The views expressed in this article, are those of the authors and not necessarily those of Topcon.
References
- G. Coscas et al., “Optical Coherence Tomography Angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration. A new diagnostic challenge”, Retina, 35, 2219–2228 (2015).
