
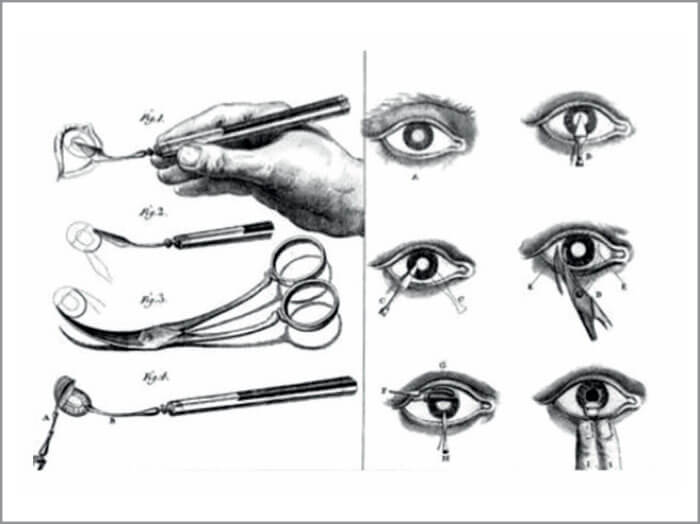
The capsulotomy has a fascinating history – one tinged with jealousy, rivalry, fashion, evolution, revolution and even nationalistic pride – and it’s a story that’s got a great deal more to tell. It started with the Frenchman, Jaques Daviel, who ushered in the modern era of cataract surgery, abandoning couching (Box 1) for the first extracapsular cataract extraction (ECCE; Box 2). The British, for predominantly patriotic reasons, preferred couching (Box 3), but ECCE was always going to win out. Albrecht von Graefe improved it in 1850 with his eponymous knife (Box 4) and, extraordinarily, his technique lasted for over a century. That’s not to say that competing approaches weren’t developed along the way – Ignacio Barraquer devised the suction cup-based erisophake in 1917, which enabled rapid intracapsular extraction of the lens (Box 5), but its uptake was limited. So the von Graefe method persisted into the 1970s.
The late 1950s and early 1960s saw lensectomy return to an intracapsular approach, thanks to a combination of Joaquin Barraquer’s enzymatic zonulolysis approach and cryoextraction of the lens (Box 6). Around that time, Cornelius Binkhorst, one of the pioneers of lens implant surgery, realized that to make intraocular lenses (IOLs) work, you needed to move away from fixation to vital tissues and use the capsule instead. And that’s why he developed his two-loop iridocapsular lens – and he tried many different shapes of capsulotomy to get the best fixation (Box 7). We all know the story of how Charles Kelman introduced phacoemulsification in 1967 – and Charlie devised a new way to make the capsulotomy, using a hooked cystitome: the Christmas tree (Box 8). However, Kelman was performing anterior chamber phaco – and, to do that, you need to prolapse the nucleus into the anterior chamber. Dick Kratz decided that he would like to perform phaco more in the posterior chamber, so he came up with the “can opener” capsulotomy in the 1970s, which resulted in a ‘roundish’ opening (Box 9).
Time for CCC
To begin with, the capsular opening was simply to give access to the cataract, but IOLs changed that. I remember being at the AAO Annual Meeting in 1983 when there was a vigorous debate between the people who designed these lenses: Steve Shearing, Bob Sinskey and Dick Kratz, as to whether or not the lens should go into the capsular bag or the sulcus. The reason for the debate? You really couldn’t guarantee that you’d be able to keep the IOL in the bag with the can-opener capsulotomy. But of course, this all changed with the development of the continuous curvilinear capsulorhexis (CCC), which is essentially the current state of the art in (manual) cataract surgery (Box 10). The CCC meant that IOLs could be placed reliably and securely in the capsular bag – although it did mean that certain procedures needed modification because of the containment by the capsular opening. And indeed they were. Several new phaco techniques were developed to overcome these difficulties: chip and flip, divide-and-conquer, nucleofractis and phaco chop and prechop. We know that one of the problems with leaving the posterior capsule in place (particularly in children) is that you get posterior capsule opacification (PCO). Howard Gimbel gave us this technique to do a posterior capsulotomy and then capture the IOL optic in it. Daniele Aron Rosa gave us the YAG laser back in 1982, which wasn’t practicable for anterior capsulotomies (Box 11) – but it reliably performs posterior capsulotomies, and is a standard procedure today for dealing with PCO. The challenge has always been to make the CCC more precise in terms of size and centration, as this helps with IOL centration, stability, effective IOL lens position, and being able to overlap the edge of the IOL with the capsule and “shrink-wrapping” the lens to lessen PCO. These are important factors, but acutely more so when multifocal and toric IOLs are used. Then came the femtosecond laser. First used clinically by Zoltán Nagy in 2008 (Box 12), it represented another fundamental change in how capsulotomies are made. For the first time, we could make a truly circular capsulotomy of a pre-specified size in a pre-specified position – without the variables of the manual technique. But it did come with some caveats. You might need a second room for your laser, which might interfere with your surgical flow. The device purchase and running costs are high, and as the recent EUREQUO registry publications have shown, there are very few scenarios where patients have derived any benefit over manual cataract surgery where the surgeon performs a CCC, rather than the laser performing a capsulotomy. Might there be other ways of achieving the same consistency and accuracy? If you’re performing CCCs by hand, you might consider Marie-José Tassignon’s CCC ring; once centered on the capsule, you tear inside it. Others simply mark the cornea – or use the high-tech equivalent, having it rendered on a head-up display (like Alcon provides with Verion), which can be particularly useful when you get a widely dilated pupil or a very large eye. However, on the horizon, there are a number of new approaches that make the capsulotomy more accurate and precise.Box 1. Couching Stabilize the eye with your thumb; introduce the needle perpendicularly into the eye, entering three millimeters behind the limbus. Gradually push forward with a rotating movement. Move the needle tip downwards to disrupt the lower zonule. Push the lens down into the vitreous with some slow sweeping movements. At least it is minimally invasive…
Box 2. Daviel’s ECCE technique for opening the capsule and extracting the nucleus, first described in 1747 Daviel used a keratome and a pair of scissors to create a corneal flap, a cystitome to “circumsize” the capsular bag, a blunt spatula placed between the lens and iris to free the lens – and two fingers, gently pressed on the lower lid to express the nucleus.
Box 3. The UK vs. Europe Given “Brexit”, you might not be surprised to learn that the British medical establishment were very skeptical of Daviel’s new ECCE technique, when it was introduced. Percival Pott, a surgeon at St. Bartholomew’s Hospital, known for Pott’s disease (tuberculosis of the spine) and Pott’s fracture of the ankle, decided to publish in 1771 that, as far as he was concerned, cataract extraction was just a passing fashion, and he would continue to advocate couching the lens. Their European colleagues, of course, viewed things differently. Austrian surgeon, Joseph Beer, said, “Some of the English ophthalmologists rejected the extraction method in order to please Mr. Pott, others in order to stand out among the crowd. A third group did it out of national pride and out of hate of all things French. And a fourth group did it because they had bad results due to clumsiness.”

Box 4. Von Graefe sets the standard for more than a century In 1850, Albrecht von Graefe refined the corneal flap procedure using his eponymous knife, and introduced a peripheral linear incision superior to the cornea; the shift in location from 6 o’clock to 12 o’clock further meant that the eyelid protected the wound, reducing infection and halving the failure rate from 10 to 5 percent.

Box 5. Ignacio Barraquer debuts the erisophake in 1917 Anesthesia, incision, application of the erisophake (a special cup and suction apparatus) then removal of both lens and instrument made for a very rapid procedure. Many surgeons visited Barraquer to learn the technique, but most continued to perform von Graefe’s method.

Capsulotomy’s future Capsulaser
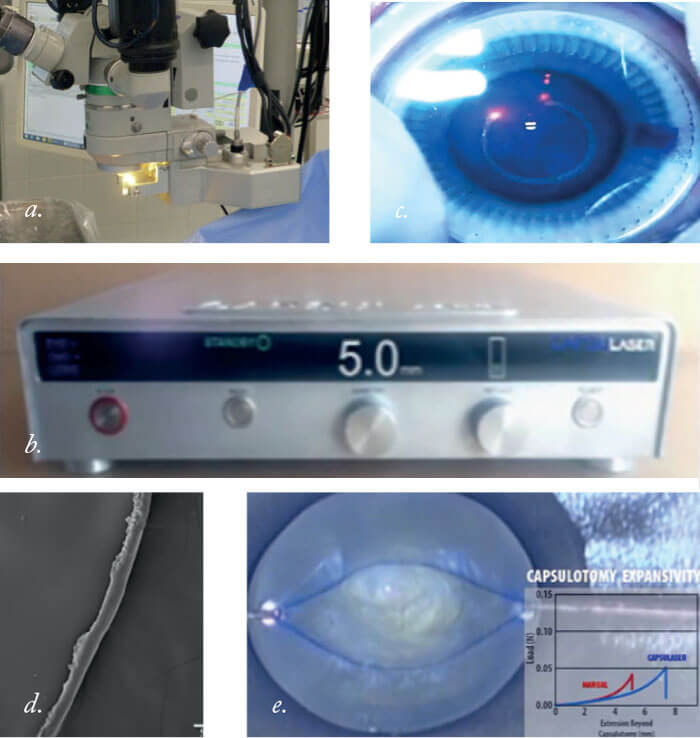
The Capsulaser device (Excel-Lens; Box 13) is a small laser that is attached under the surgical microscope, and is connected to a small, shoebox-sized console. It’s a continuous laser, rather than a pulsed laser, and it scans in a single circular pattern over a period of one second to create the curvilinear capsulotomy opening. It requires the anterior capsule to be stained with Trypan blue, which creates a chromatically selective target for the laser. The irradiation changes collagen IV to elastic amorphous collagen, and this phase change results in not only a smooth edge but also a very elastic capsular rim (the capsulotomy can be extended from 5 to 12 mm without fracture or tear). And thanks to how the laser works, it’s almost impossible not to get a free cap. The preliminary results have been good, there’s been no pupil construction after laser use, no untoward anterior chamber activity postoperatively, and in a case series of 20 patients, their corneas remained clear out to 18 months, with similar endothelial cell counts to patients that underwent normal cataract surgery. Also noteworthy is that these capsulotomies have remained well centered and have not contracted at all – meaning that there’s been no subsequent change in IOL position. To date, another 400 eyes have been operated upon and the CE mark trial has recently been completed. The device has also been used to perform posterior capsulotomies in cadaver eyes, and what’s interesting here is that it leaves the anterior hyaloid intact.Zepto
There are also now thermomechanical devices being developed for anterior capsulotomy: you are probably aware of the Zepto device (Mynosys; Box 14). It consists of a soft, foldable clear suction cap that contains a nitinol capsulotomy ring that’s inserted through a 2.2 mm clear corneal incision using a disposable handpiece. Once in the eye and aligned over the visual axis, a push rod is retracted and the device unfolds into a circular shape, whereupon the surgeon can center the device and apply suction. A pulse of electrical energy is delivered in milliseconds to the nitinol ring, triggering a rapid phase transition of water molecules that are trapped between the device and the capsular membrane – instantaneously creating a cleavage plane in the capsular membrane, making a strong capsulotomy. It is CE-marked and now available in Europe.ApertureCTC
Mark Packer has been involved with ApertureCTC (International Biomedical Devices; Box 15) – another thermal device, which is currently under preclinical evaluation. It’s similar to Zepto in many respects, but lacks the suction cup. It consists of a reusable handpiece and a disposable 1.2 mm diameter tip that houses a circular 5.25 mm filament, which can be deployed once the tip is in situ. This is gently depressed on the capsule, a foot pedal is used to deliver a millisecond pulse of thermal energy, which melts the collagen and creates a perfectly round capsulotomy – and with it a smooth, strong and elastic capsular opening. The manufacturers report that multiple filament dimensions are available, ranging from 4–6 mm.Capsulotomy’s evolution
The role of the capsulotomy has changed with cataract surgery over the last 270 years – from a crude opening to give access to the nucleus for its removal, through various iterations of cystitomes and forceps to create different shapes of opening for IOL support, to CCC for those anterior and posterior capsules to contain the IOL. And now we have many devices from lasers to metallic thermal instruments to automate the creation of perfectly round consistent central capsulotomies – which should offer more effective lens position predictability.Box 6. Joaquim Barraquer discovers alpha-chymotrpysin’s ability to dissolve zonules (1958) This, combined with Tadeusz Krwawicz’s cryo probe (1961) – and its subsequent improvements by Percy Amoils (1965) – turned the tide back to intracapsular cataract extraction.
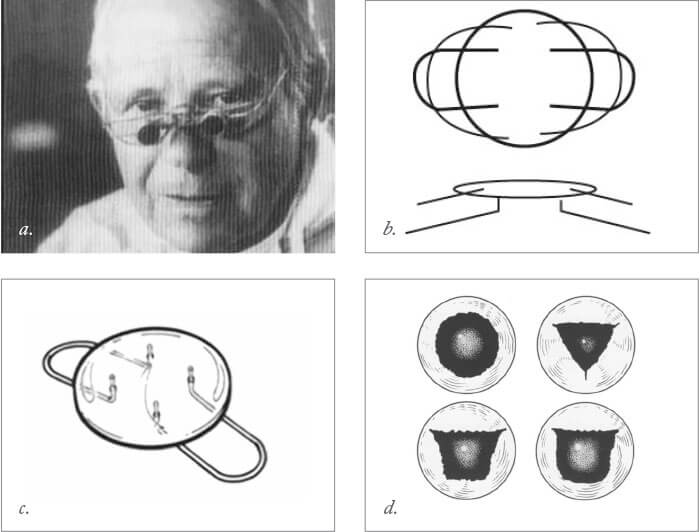
Box 7. Binkhorst’s experimentation Cornelius Binkhorst (a) realized that good fixation away from vital tissues was critical, designed the iris-fixated four-loop lens (b) for use after intracapsular surgery. However, Binkhorst felt that if he could fixate a lens in the capsular bag it would remove the need for pupil involvement. This led to his two-loop iridocapsular IOL (c) – and he tried lots of different shapes of capsule opening to achieve best fixation (d).
Box 8. Charlie Kelman’s Christmas Tree Kelman introduces phacoemulsification in 1967 – and devises a new way to do capsulotomy with a hooked cystitome: the “Christmas tree.”

Box 9. Dick Kratz’s can-opener approach Dick Kratz decided that posterior chamber phaco was safer than in the anterior chamber and devised the “can opener” capsulotomy and his iris plane phaco technique. One of the issues at the time was whether or not the IOL should go in the capsular bag or into the sulcus – you couldn’t guarantee that the can opener capsulotomy would keep the lens in the bag.
Box 10. The origins of the CCC Calvin Fercho from Fargo, North Dakota, started using a continuous tear capsulotomy in the late 1970s, and this was the predecessor of what became termed “continuous curvilinear capsulorhexis” (CCC), which was developed and taught by Howard Gimbel, Thomas Neuhann and Kimiya Shimizu in the mid-1980s. The CCC significantly reduced intraoperative complications, such as anterior capsular rim tear, and improved IOL centration and sequestration in the capsular bag. The arrival of Peter Utrata’s eponymous forceps in 1988 helped make CCC more controllable and easier to perform for many surgeons.

Box 11. The YAG laser Daniele Aron Rosa tried her newly invented YAG laser for anterior capsulotomies back in 1982. The main drawback was that the surgery had to be carried out immediately – the soft lens matter “fluffed” up and IOP rose greatly after application of the laser.

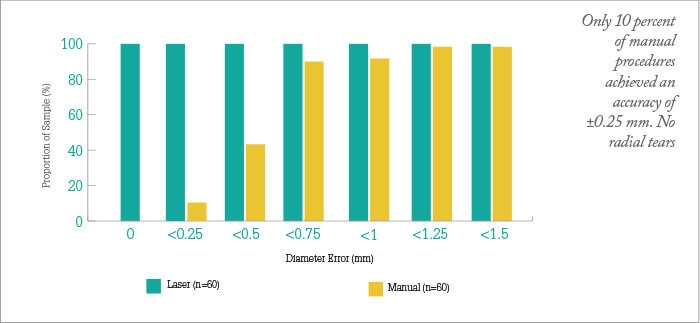
Box 12. Zoltán Nagy performs the first capsulotomy with a femtosecond laser in 2008 But how does the femtosecond laser compare with manual capsulorhexis for accuracy? In one study, 100 percent of LenSx procedures achieved an accuracy of ±0.25 mm.

Box 13. The CapsuLaser device (a) Present on the surgical microscope; (b) the console; (c) the process of laser capsulotomy; (d) the smooth edge of the disk; (e) Stronger capsulotomy than a manual CCC.
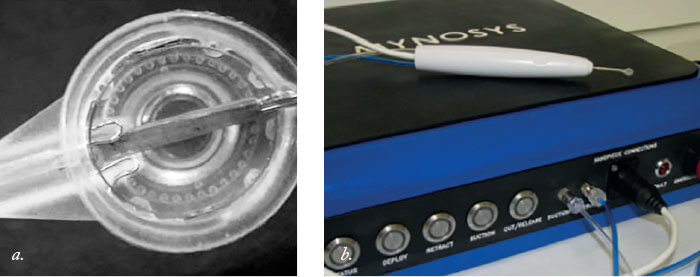
Box 14. The Zepto capsulotomy system The disposable capsulotomy tip (a) consists of a foldable, soft, clear suction cup containing a nitinol ring that can enter through a 2.2 mm incision, and expands to create a 5.2 mm circular capsulotomy. The tip is delivered through a disposable handpiece, pictured in (b) above the device’s console.
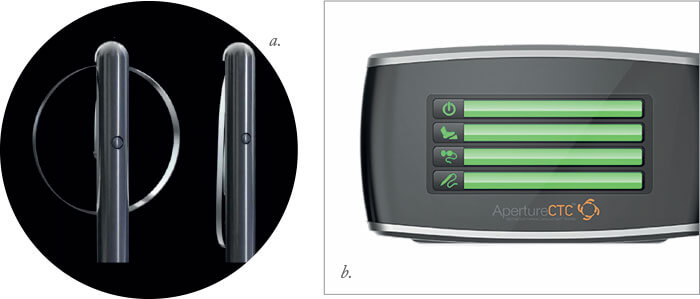
Box 15. ApertureCTC Similar to Zepto, but without the suction ring, the disposable tip (a) is intended to be introduced through a 1.8 mm incision, and will come in sizes between 4.5 mm and 6.5 mm (in 0.5 mm increments), placed on to the capsule and activated to make the thermal capsulotomy. A render of the device’s console is shown in panel (b).
Of course, IOLs have evolved with the capsulotomy, and a new generation of IOLs are being developed take advantage of this; for example, the Oculentis FEMTIS IOL with its small flaps – “rhexis clips” – that lock the lens inside the capsulotomy; or the Masket ND IOL (Morcher), which has a groove that fits inside the anterior capsulotomy. Could it be that the combination of these new, lower cost approaches to capsulotomy – plus a new generation of IOLs that rely on the properties of capsulotomies made by these devices – will soon consign manual CCCs to the history books too? Richard Packard is a consultant ophthalmic surgeon and Director of Arnott Eye Associates in London, England, and was the senior surgeon at the Prince Charles Eye Unit, King Edward VII Hospital in Windsor, and is credited as “almost single-handedly removing phaco from the blacklist in the UK giving the procedure credibility and validation.” He implanted the world’s first foldable soft lens in rabbits and has lectured and operated in 59 countries – often with Charlie Kelman, and has chaired the ESCRS’ video competition panel since 2000. He reports that he is a consultant and equity holder in Excel-Lens, and consults for Core Surgical and Shire. This feature is adapted from the presentation, “The capsulotomy from there to where? The story unfolds…” given at the Cataract Surgery: Telling It Like It Is! annual meeting, held in Naples, FL in January 2017.
