Glaucoma surgeons joke that, “If all else fails, soon we’ll be able to give our patients brain implants to keep them seeing.” They have a point – if optic nerve function is lost, then no retinal prosthesis is going to help. Indeed, if a retina is so damaged by disease that its bipolar and ganglion cells fail to function properly, it’s clear that no “bionic eye” could ever help. And so ever since the 1920s (1), people have been working towards a visual prosthesis for the visual cortex of the brain.
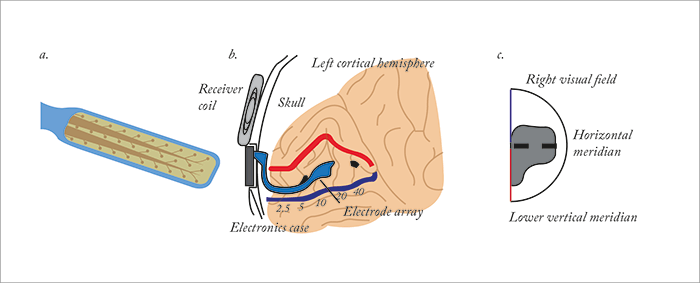
Nearly 100 years later, Second Sight recently reported the “first successful implantation and activation of a wireless multichannel visual cortical stimulator in a human subject, providing the initial human proof of concept for the ongoing development of the company’s Orion I visual cortical prosthesis, Orion I” (Figure 1). There are a number of good reasons why it’s taken almost a century to get visual cortical prostheses to this stage. Placing the implant requires craniotomy, and once you’ve removed a portion of the skull to gain access to the brain, you have to deal with a brain anatomy that’s both distorted relative to the visual input, and plastic to neural input. In other words, even if you can safely place it, can you “tune” it and keep it tuned over time without requiring repositioning? There are also worries over “kindling” – causing a seizure with electrical stimulation and making its recurrence more likely. However, there’s around 5.8 million people in the world today who are legally blind who could be treated with a visual cortical prosthesis, so getting it right should help a great number of people regain at least some form of vision. It’s reported that the first 30-year-old patient to receive the Orion I device “was able to perceive and localize individual phosphenes or spots of light with no significant adverse side effects.” We asked Second Sight’s Vice President, Gregoire Cosendai about the device.
What sort of “visual bandwidth” is Orion I expected to have?
It’s too early to tell, but work by William Dobelle in the 1980s showed that reading was possible, at least to a levels that’s as good as the Argus II.Have you any idea of the optimal placement of the external camera?
In the center. We have data to support that this is the best choice.What sort of daily tasks do you expect a patient with an implanted Orion I to be able to do that they couldn’t do when blind?
We expect them to find objects, follow lines, conduct orientation and mobility – similar to the Argus II initially, but again, it’s too early to tell.Could multimodal imaging work, like adding in the infrared spectrum, to highlight a boiling kettle, for example?
Yes. We are doing this in the next generation Argus II and we will have it in the Orion too – the thermal imaging highlights people, for example.The anatomy of the primary visual (V1) cortex is distorted relative visual input (i.e. a grid array of electrodes won’t give the patient the perception of a grid of phosphenes). How does the Orion cope with that?
It’s distorted, for sure, but the area of central vision will be mapped to “squeeze” the visual field in the right place.We all know that the cortex is plastic even in adults, and Brindley and Lewin’s work has shown the optimal settings for the electrodes in the array change over time. How is the Orion I able to cope with that?
We hope the cortex will adapt positively to vision. Our implant is designed to be adjusted by software; we hope plasticity will work in our favor as it did for Argus II.Given how the brain adapts, can the Orion I ever be replaced or repositioned?
Probably, but we hope this will not be necessary.What size of craniotomy is required, and what sort of risk and recovery times are involved?
Minimally invasive. The device will not penetrate the brain. Craniotomy will be about 10 mm. As always, safety is our top priority.Kindling has occurred with previous attempts at cortical visual prostheses. How does the Orion I minimize/avoid this risk?
It’s one of the things we want to evaluate in the clinical trial. Our device has very precise current control.What are the costs likely to be, in terms of both device and surgery?
It’s too early to speak about cost. But in terms of health economics, we can say that the cost of blindness is 5-10 million Euros over the lifetime of a patient…References
- O Foerster, “Beitriige zur Pathophysiologie der Sehbahn und der Sehsphare”, J Psychol Neurol, 39, 463-485 (1929). United States Securities and Exchange Commission. Second Sight Medical Products, Preliminary Prospectus. Available at: http://bit.ly/2f96W9W. Last accessed October 31, 2016.
