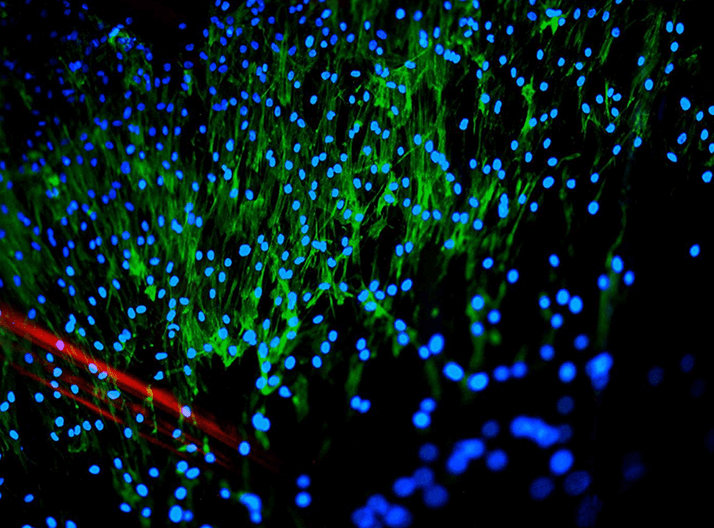
Stem cell therapies hold tremendous potential for treating ophthalmic disease, but as the field is still relatively young, many questions remain unanswered. One research team, led by staff from St. Jude Children’s Research Hospital, set out to discover more about the biology of stem cells by posing a question: how do you identify the best stem cell source for transplantation into the retina?
The team examined three stem cell types: embryonic stem cells (ESCs), fibroblast-derived induced pluripotent stem cells (f-iPSCs), and iPSCs derived from murine rod photoreceptors (r-iPSCs), all of which are able to produce retinal pigment epithelium (RPE) in culture. The different types of cells produced were then compared using the STEM-RET protocol, which quantifies retinogenesis using measurements of molecular, cellular and morphological criteria. The researchers found that r-iPSCs are more efficient at producing differentiated RPE, whereas f-iPSCs show reduced numbers of inner nuclear layer and ganglion cell layer cells. “There has long been a debate in the field about how to standardize the quantification of stem cell differentiation,” says Michael Dyer, lead author of the study (1), adding, “Our STEM-RET method enables that standardization, which means that laboratories can accurately compare their results with one another and different stem cell lines can be compared. We believe the method could be adopted widely.” But why might r-iPSCs make superior RPE cells? One factor might be epigenetic memory – the photoreceptor-derived cells retain distinct epigenetic switches after being reprogrammed, which affects how well they can produce different cell types and could explain why rod-derived stem cells are better than other types at creating differentiated retinal cells. Dyer hopes that using this information to create epigenetic “fingerprints” could allow for better selection of cells for therapeutic purposes.
References
- D Hiler, et al., “Quantification of retinogenesis in 3D cultures reveals epigenetic memory and higher efficiency in IPSCs derived from rod photoreceptors”, Cell Stem Cell, 17, 101–115 (2015). PMID: 26140606.
