- The pathophysiology of many posterior segment diseases involves the choroid
- Historically, imaging the choroid has been particularly challenging
- EDI-OCT produces detailed, high-resolution images of the choroid and the outer part of the globe
- Will help diagnosis, staging and prognostic predictions of these diseases
While optical coherence tomography (OCT) was revolutionizing retinal imaging over the last decade, the choroid remained hidden from view. Now, a new approach, called enhanced depth imaging (EDI)-OCT, is defining choroidal structures in a way that users of earlier techniques, such as ultrasound and indocyanine green (ICG) angiograms, could not have dreamed of. This is a noteworthy development. The choroid is the main vascular layer of the eye, providing nutrition and support to the retina in front of it; choroidal dysfunction, understandably, underlies many of the pathophysiologies of posterior segment diseases. Obtaining good images of a patient’s choroid is critical for the diagnosis and understanding of diseases of the choroid.
Evolving OCT
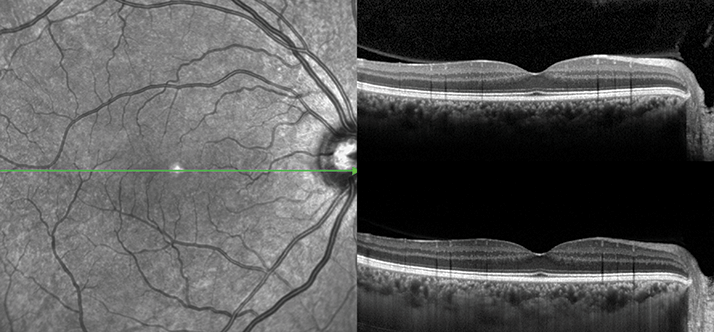
The choroid is almost invisible to conventional OCT, an interferometric technique that relies on capturing three-dimensional images from tissues that scatter the light. Lying just under the retinal pigmented epithelium (RPE), the choroid simply reflects most of the laser light shone at it. How has the technology been adapted to deal with this? OCT requires image processing to provide the final, resolved picture and a number of light scanning and imaging processing techniques have been developed over the years, the original being time domain (TD)-OCT. An important concept in EDI-OCT imaging is the ‘zero-delay line’ – a reference point determined by the imaging processing method and software at which the best image is captured. In conventional OCT, the zero-delay line is usually at the inner surface of the retina, which is far from ideal for imaging the choroid.Spectral domain (SD)-OCT advanced things by improving pixel densities and signal-to-noise ratios – essentially enabling more to be seen. With SD-OCT, structures that are closer to ‘zero delay line’ emit more signal than structures farther away. EDI was a simple evolution of SD-OCT – the imaging apparatus was placed slightly closer to the patient’s eye, resulting in the ‘zero delay line’ moving over the outer retina and choroid. This, in combination with more advanced image processing software has finally permitted high-quality images of the choroid (Figure 1).
This is not to say EDI-OCT provides a choroid-imaging utopia. Numerous factors can affect the quality of output, such as:
- Ocular wall distortions
- Large and elevated lesions at the posterior pole
- Thick choroid or thick lesions that cause loss of signal penetration and intensity
- Choroid disorders, like polypoidal choroidal vasculopathy (PCV) and central serous chorioretinopathy (CSCR), that result in significant variations in choroidal thickness
- Media opacities
- Ocular surface diseases.
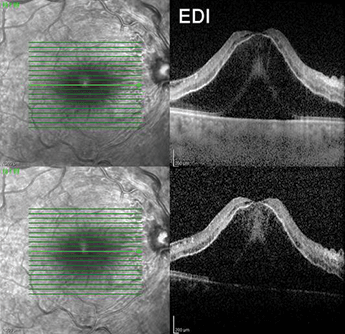
For the most part, however, EDI-OCT clearly defines choroidal anatomy and the vessel map. In cases where the patient has a thin choroid, for instance, associated with aging and myopia, sclera and even large penetrating vessels may be visible. Thicker choroids are associated with younger ages, tumors, CSCR, hyperopia, and choroiditis. Where evaluation of the deeper retinal structure is required, such as in the case of retinal edema, EDI-OCT can achieve this (see Figure 2).
Clinical applications
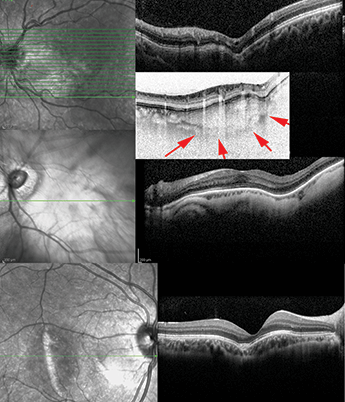
Choroidal measurements Among EDI-OCT’s assets are that it permits assessment of choroidal thickness and volume and enables the mapping of choroidal thickness, which can be used to assess the extent of disease-related changes. There are some drawbacks – measurement of choroid thickness is less precise than measurement of retina thickness, in part because choroidal thickness varies more. In addition, accurate definition of the chorioscleral interface can be tricky since the outer boundary of the choroid might be imprecise; this may explain the wide variation in choroidal thickness among recent reports (1). This issue has been resolved, and if you’ve held a bright, white-light torch to your hand, you’ll know how. Longer wavelengths (red light) penetrate tissue better than shorter wavelengths. This principle applies with OCT, and has been exploited in high penetration OCT (HP-OCT) which uses longer-wavelength source light for deeper choroidal imaging. Both EDI- and HP-OCT provide consistently reproducible choroidal thickness measurements. Choroidal tumors An important application of EDI-OCT is the diagnosis and monitoring of choroidal tumors. The level of image detail is far in excess of other methods -EDI-OCT can clearly identify the tumor boundary from the surrounding normal choroid. Interesting anatomical changes in choroidal metastasis have been revealed, such as the cauliflower-like irregularities on the tumor surface and an abnormal hyper-reflective RPE. Additionally, EDI-OCT permits more precise measurements of small choroidal melanoma thickness. A recent study found that, in comparison, ultrasound techniques overestimate this by 55 percent. The technique is powerful enough to show the differences between small choroidal melanoma and simple choroidal nevus, including increased tumor thickness, subretinal fluid, subretinal lipofuscin deposition, and retinal irregularities – including shaggy photoreceptors. EDI-OCT is a useful tool for the monitoring of tumor size after treatment and can also differentiate between choroidal tumors and ocular wall distortions like posterior staphyloma or choroidal atrophy secondary to trauma or inflammation (see Figure 3). For these reasons, it is recommended that EDI-OCT is performed on every patient with ocular wall distortion in conventional OCT images to rule out choroidal mass or tumor. However, the outer boundary of choroidal layer needs to be verified in such cases in order to avoid missing the choroidal tumors.Glaucoma EDI-OCT has revealed a number of new anatomical changes in glaucomatous eyes, such as the lamina cribrosa being located more posteriorly at its central and midperipheral parts, and focal loss of Laminar beam or even acquired pitting of the optic nerve (in advanced cases). The technique has revealed alterations and lesions to structures within the deep optic nerve complex of patients with glaucoma. As age-related choroidal atrophy is also a risk factor for glaucoma, EDI-OCT of both the choroid and the optic nerve might prove to be an excellent diagnostic and prognostic imaging modality for patients with glaucoma in the future. Uveitis The choroid is the origin of most posterior uveitis and intraocular inflammation. One well-described intraocular inflammatory disease is Vogt-Koyanagi-Harada (VKH) syndrome; it is characterized by thickening of the choroid, which is partly related to both inflammatory infiltration and exudation. VKH syndrome is treated with chronic corticosteroid therapy, and the length of time that the drug dosage should be tapered and discontinued is an area of debate as corticosteroids decrease choroidal thickness. Evaluation of choroidal structure and thickness is helpful in assessing treatment efficacy or the recurrence of the disease, so EDI-OCT could prove useful for managing and diagnosing VKH syndrome. Choroidal thickness may also serve as a marker for the degree of inflammation in VKH syndrome; EDI-OCT studies in patients with VKH revealed a significant loss of focal hyperreflectivity in the inner choroid in the acute and convalescent stages of the disease, with the choroid being thicker in the acute-phase than the convalescent-phase (5). Nevertheless, in long-standing VKH, progressive choroidal thinning is evident. There have been few reports of EDI-OCT applied to patients with other forms of intraocular inflammation. One case of multifocal choroiditis has been published (6), reporting localized thinning of the choroid, occlusion of the choroidal vessels, and localized hyper-reflectivity that may represent hyper-pigmentation of the choroid.
Summary
Choroid dysfunction plays a large role in the pathophysiology of many posterior segment diseases. The development of EDI-OCT and its derivatives is a significant technological advance that has greatly improved our understanding of the pathophysiology behind many of the diseases that affect the choroid. Beyond this, EDI-OCT is beginning to be used to improve diagnosis, stage and prognostic predictions of choroidal diseases. It has become a valuable addition to the ophthalmologist’s diagnostic armamentarium. I look forward to further enhancements of this relatively young imaging technique, and the benefits that these will bring.Fedra Hajizadeh is a Vitreoretinal Consultant and Ophthalmology Surgeon at the Noor Eye Hospital, Tehran, Iran.
References
- R. Margolis and R. F. Spaide, “A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes”, Am J Ophthalmol., 147, 811–815 (2009). G. Querques, R. Lattanzio, L. Querques, et al., “Enhanced depth imaging optical coherence tomography in type 2 diabetes”, Invest Ophthalmol Vis Sci., 53:6017–24 (2012). S. W. Kim et al., “Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy”, Retina, 31, 1904–1911 (2011). L. Yang, J. B. Jonas, W. Wei, “Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy”, Invest Ophthalmol Vis Sci., 54, 4659–65 (2013). A. H. Fong, K. K. Li, D. Wong, “Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease”, Retina, 31, 502–9 (2011). Y. Yasuno, F. Okamoto, K. Kawana, et al., “Investigation of multifocal choroiditis with panuveitis by three-dimensional high-penetration optical coherence tomography”, J Biophotonics, 2, 435–41 (2009).
