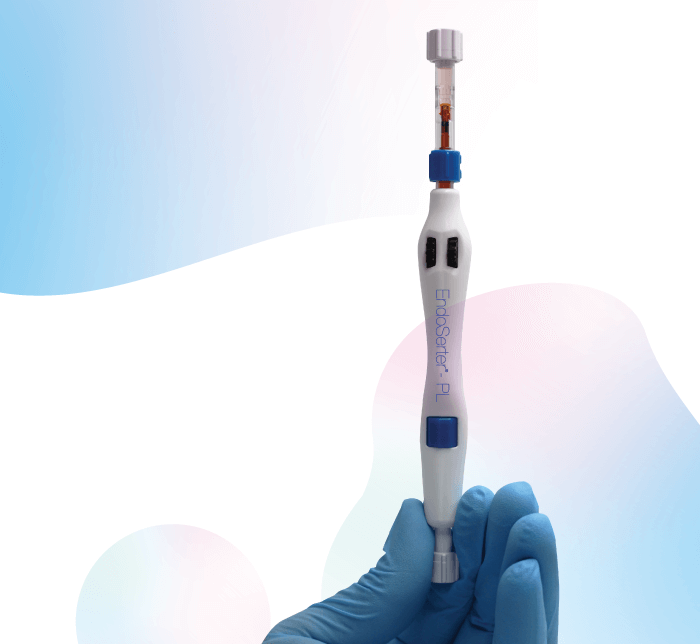
CorneaGen first released its EndoSerter Corneal Endothelium Delivery Instrument in 2011, providing surgeons with a sterile, single-use device that offered a controlled method of delivering and positioning the donor allograft into the anterior chamber of the eye during Descemet’s stripping endothelial keratoplasty (DSEK). Donor tissue is inserted through a tight 4 mm clear corneal incision, reducing the need for sutures.
The EndoSerter’s intelligent design allows coaxial irrigation, eliminating the need for a separate chamber maintainer. Unlike conventional insertion devices, the EndoSerter does not push or pull on the fragile allograft tissue and endothelial cells; the allograft is simply uncovered during deployment.
Unsurprisingly, the EndoSerter was well-received by the ophthalmic community – and expectations are high for its successor. The latest device, the preloaded EndoSerter-PL, has been designed with automated procedures in mind – procedures which now represent the largest segment of all domestic corneal transplant cases in the US. The all-new EndoSerter-PL streamlines the process by acting as both a shipping container and insertion device. CorneaGen processes the tissue to the surgeon’s exact specifications and loads the graft into the EndoSerter-PL, saving precious time in the OR. In short, the preloaded EndoSerter-PL simplifies the DSAEK surgical procedure – better serving surgeons (improved procedure) and, ultimately, better serving patients.
But the service doesn’t stop there. CorneaGen works directly with each surgeon to identify their graft thickness needs. In response to demand for everthinner tissue, CorneaGen has developed Nano-Thin™ DSAEK – at less than 60 microns, it is the thinnest tissue on the market and a perfect accompaniment to the EndoSerter-PL, and just one example of how CorneaGen continues to pioneer the next generation of cornea care.
From new medical devices and biologics to therapeutics and interventions, CorneaGen continues to support corneal surgeons and their patients in the fight against preventable blindness. Available spring of 2021, the release of the newly refined preloaded EndoSerter-PL brings the company one step closer on its mission to eliminate corneal blindness by 2040.
