- Normally, CXL should only be performed on eyes with a minimum corneal thickness of 400 µm – any thinner risks UV damage of the deep stroma, corneal epithelium and beyond
- Thinner corneas might be able to be treated using a number of alternative methods (like swelling the cornea with hyperosmolar riboflavin solution) – but drawbacks exist, and treatment failure can still occur
- Tailored stromal expansion with a lenticule extracted from another patient who underwent a SMILE procedure for myopia could offer a safer, more effective way of administering CXL to thin corneas
- Though the method still needs further refinement and longer-term studies in larger patient populations, it has shown promise in early test cases
- Normally, CXL should only be performed on eyes with a minimum corneal thickness of 400 µm – any thinner risks UV damage of the deep stroma, corneal epithelium and beyond
- Thinner corneas might be able to be treated using a number of alternative methods (like swelling the cornea with hyperosmolar riboflavin solution) – but drawbacks exist, and treatment failure can still occur
- Tailored stromal expansion with a lenticule extracted from another patient who underwent a SMILE procedure for myopia could offer a safer, more effective way of administering CXL to thin corneas
- Though the method still needs further refinement and longer-term studies in larger patient populations, it has shown promise in early test cases
Keratoconus is a non-inflammatory progressive corneal thinning and ectasia of unknown etiology in which the cornea assumes a conical shape. It is associated with irregular astigmatism, central corneal scarring and progressive myopia resulting in impaired visual acuity (1). Although there are a number of treatment options – from contact lenses to keratoplasty – before the advent of corneal collagen cross-linking (CXL) with UV light and riboflavin, none were able to alter the natural course of the disease (1,2). CXL, however, has shown that it has the potential to be disease-altering; it acts to increase the cornea’s biochemical strength and can slow – or even halt – the progression of the disease (3,4,5). Nevertheless, it is not a panacea – there are limitations on what the technique can achieve and on whom.
Currently, the most effective form of CXL is the Dresden protocol (3) – photosensitizing the cornea with iso-osmolar riboflavin (0.1% solution in 20% dextran) for half an hour and then exposing to UV-A radiation (370 nm, 3 mW/cm²) for an additional half hour. However, it’s important to note that the effects of the riboflavin and the UV-A irradiation are restricted to the anterior ~300 µm of corneal stroma (3,6,7). Continual application of riboflavin and a stromal thickness of at least 400 µm are both critical to the procedure, as the combination of the two prevent the UV-A irradiation used during the process from penetrating into sensitive ocular tissues like the deep stroma, corneal endothelium or even the crystalline lens (6,8,9). But one of the hallmarks of keratoconus and other corneal ectasias is the thinning of the cornea, and this means that many patients with advanced disease have corneas that are too thin for safe cross-linking. One method is to swell the corneal epithelium with hypo-osmolar riboflavin beforehand, bringing its thickness to the minimum 400 µm, as described by Farhad Hafezi’s group (10). However, this procedure is less effective than the Dresden protocol CXL, and this might be due to artificially swollen corneas not behaving like non-swollen ones during the procedure. It’s also possible that the decreased relative collagen concentration in the stroma makes CXL less effective – and Hafezi’s group suggest that taking this approach in any cornea thinner than 330 µm may be inadvisable (11).
Another option is transepithelial or “epi-on” CXL, which was introduced to prevent the complications associated with epithelial debridement – principally pain, haze and an increased risk of infection. Though researchers have reported statistically significant improvements in visual and topographic parameters (12,13) with epi-on CXL, the current evidence shows that the traditional epi-off methods are still more effective, and endothelial cell toxicity associated with the transepithelial solutions used remain a concern. Recently, Kymionis et al. (14) performed customized pachymetry-guided epithelial debridement in two patients with progressive keratoconus. They performed a central 8 mm epithelial debridement, but preserved a small localized island that corresponded to the thinnest or steepest area. Although this technique has some advantages – preventing local stromal dehydration, blocking excess UV-A in the most sensitive region – it fails to strengthen the thinnest regions that most require cross-linking, and concerningly, an anterior segment optical coherence tomography (AS-OCT) and confocal microscopy study has demonstrated stromal haze and the demarcation line in areas corresponding to de-epithelialized stroma – things that were not evident in areas with an intact epithelium (15). Finally, a highly innovative approach has also been suggested is the use of riboflavin-soaked bandage contact lenses to artificially increase the corneal thickness for CXL (16). Unfortunately, originality has its drawbacks, as it’s impossible to customize the thickness of the lens, different materials have varied hydration states and UV-A transmission properties, and the lenses may adhere unevenly to the stromal bed, causing the riboflavin to pool.
Sadly, what this means that there’s a significant population of patients with corneal ectasias that have progressed beyond the point where they are able to receive a truly effective intervention – CXL. It’s an issue I’ve encountered in my own practice, and one that’s prevalent in many developing countries. It’s also a particular problem in Asian countries, where keratoconus arises earlier and is diagnosed later – so many of our patients have already progressed beyond the limits for safe CXL. This might not be the case for much longer. We have devised a new method for increasing the intraoperative corneal thickness: using refractive lenticules extracted from eyes undergoing small incision lenticule extraction (SMILE) surgery for myopia (without astigmatism).
How do we do it?
SMILE is a novel refractive surgery that involves the extraction of a femtosecond laser-constructed corneal lenticule through a single small incision without raising a flap (17). The lenticular thickness depends upon the refractive error of the patient, but is greatest at its center and decreases toward the periphery (18). We’ve devised a new technique that takes advantage of the extracted lenticule, which is added onto the ectatic corneal surface after epithelial debridement to tailor stromal expansion for performing CXL in thin and ultrathin corneas. Using a lenticule allows us to increase corneal thickness by adding tissue with the same biological and absorptive properties; we can also place the lenticule over the apex of the cone to augment thickness where required and enable the remaining stroma to be cross-linked normally. We performed CXL using our modified technique in seven eyes, each of which had documented progression of keratoconus with steepening on corneal topography over a period of one year. Pachymetric analysis revealed a corneal thickness of less than 400 µm at the area of maximum steepening. The procedures were planned along with SMILE surgeries on patients with moderate myopia and no astigmatism. Based on the technique described by Shah et al. (17), SMILE using a VisuMax femtosecond laser (Carl Zeiss Meditec AG) was performed under topical anesthesia. The refractive lenticule, 6.2 mm in diameter with an estimated central thickness of 110 to 120 µm, was extracted intact for use in CXL corneal augmentation – and the femtosecond cuts were so precise that the lenticule was highly likely to form a stable assembly when placed on a de-epithelialized cornea.We performed our CXL operations under aseptic precautions as follows:
- The patient’s eye is cleaned and draped.
- Proparacaine 0.5% is instilled three times at five-minute intervals, 15 minutes prior to the procedure.
- The central 8 mm of corneal epithelium is debrided with a blunt spatula.
- Intraoperative pachymetry determines the required thickness of the refractive lenticule.
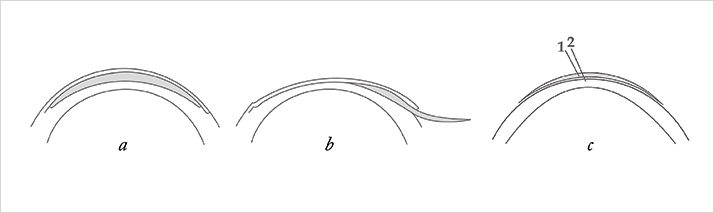
- The central area of the lenticule (corresponding to the optic zone of the donor eye) is placed over the apex of the cone so that the thinnest area of the cone corresponds to the thickest area of the lenticule (Figure 1).
- The augmented stromal thickness is confirmed to be by intraoperative ultrasonic pachymetry to be at least 400 µm.
- Instill one drop of riboflavin (0.1% solution) every five minutes for 30 minutes, using slit lamp examination to confirm the presence of yellow flare in the anterior chamber and ascertain adequate penetration of the dye.
- Apply UV-A radiation (365 nm, with an irradiance of 3 mW/cm²) at a distance of 5 cm for 30 minutes. Continue instilling one drop of riboflavin every five minutes while irradiating.
- Once irradiation is complete, peel the refractive lenticule from the stromal bed.
- Irrigate the corneal surface with normal saline.
- Apply a bandage contact lens to be removed on the fifth postoperative day.
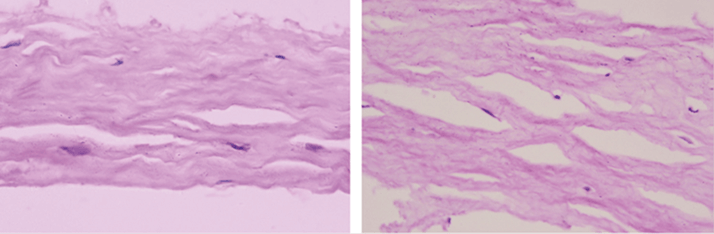
In our patients, the refractive lenticule showed increased rigidity after CXL, and histopathological examination revealed cross-linking (Figure 2). In addition to the bandage contact lens, the patients received postoperative medications including gatifloxacin 0.3% eyedrops four times daily for seven days, lotepredenolol acetate 0.5% eyedrops three times daily for 20 days, and hypromellose 0.3% eye drops six times daily for 45 days.
How did it work?
In all seven eyes, we saw an even demarcation line indicative of successful cross-linking (19). The epithelium healed completely within three to five days of the procedure, after which bandage contact lenses were removed. We noted no intraoperative or postoperative complications in any of our patients, and we were able to demonstrate corneal stability by topography at the one-year follow-up (Figures 3 and 4). Mean Kmax decreased from a preoperative value of 56.9 D, to 55.7 D at one year postoperatively. Specular microscopy revealed no significant endothelial cell loss.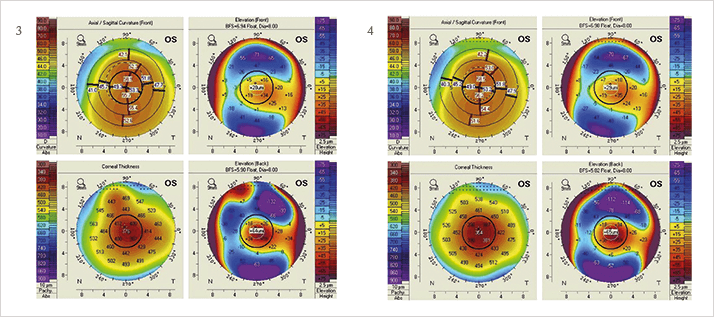
For these early procedures, we performed SMILE and CXL one after another in adjacent operating rooms to maintain sterility. A method for sterile preservation of myopic lenticules would allow them to be stored after SMILE for CXL procedures at a later date, as well as enabling more widespread use of our lenticule CXL method. Overall, the technique was highly successful in our initial test cases – but of course, we still need to conduct long-term studies to further establish the efficacy and feasibility of our procedure.
References
- JH Krachmer, et al., “Keratoconus and related noninflammatory corneal thinning disorders”, Surv Ophthalmol, 28, 293–322 (1984). PMID: 6230745. YS Rabinowitz, “Keratoconus”, Surv Ophthalmol, 42, 297–319 (1998). PMID: 9493273. G Wollensak, et al., “Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus”, Am J Ophthalmol, 135, 620–627 (2003). PMID: 12719068. E Spoerl, et al., “Safety of UVA-riboflavin cross-linking of the cornea”, Cornea, 26, 385–389 (2007). PMID: 17457183. F Hafezi, et al., “Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis”, J Cataract Refract Surg, 33, 2035–2040 (2007). PMID: 18053900. G Wollensak, et al, “Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro”, Opthalmic Res, 35, 324–328 (2003). PMID: 14688422. G Wollensak, “Keratocyte cytotoxicity of riboflavin/UVA treatment in vitro”, Eye, 18, 718–722 (2004). PMID: 14739922. E Spoerl, “Increased resistance of crosslinked cornea against enzymatic digestion”, Curr Eye Res, 29, 35–40 (2004). PMID: 15370365. L Kolozsvari, et al., “UV absorbance of the human cornea in the 240- to 400-nm range”, Invest Ophthalmol Vis Sci, 43, 2165–2168 (2002). PMID: 12091412. F Hafezi, et al., “Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas”, J Cataract Refract Surg, 35, 621–624 (2009). PMID: 19304080. F Hafezi, “Limitation of collagen cross-linking with hypoosmolar riboflavin solution: failure in an extremely thin cornea”, Cornea, 30, 917–919 (2011). PMID: 21389853. M Filippello, et al., “Transepithelial corneal collagen crosslinking: bilateral study”, J Cataract Refract Surg, 38, 283–291 (2012). PMID: 22104644. L Spadea, R Mencucci, “Transepithelial corneal collagen crosslinking in ultrathin keratoconic corneas”, Clin Ophthalmol, 6, 1785–1792 (2012). PMID: 23152657. GD Kymionis, et al., “Customized pachymetric guided epithelial debridement for corneal collagen cross linking”, BMC Ophthalmol, 28, 10 (2009). PMID: 19715585. V Kaya, et al., “Efficacy of corneal collagen crosslinking using a custom epithelial debridement technique in thin corneas: a confocal microscopy study”, J Refract Surg, 27, 444–450 (2011). PMID: 21162472. Jacob, et al., “Contact lens-assisted CXL for thin corneas”, J Refract Surg, 30, 366–372 (2014). PMID: 24972403. R Shah, et al., “Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery”, J Cataract Refract Surg, 37, 127–137 (2011). PMID: 21183108. E Tay, et al., “Refractive lenticule extraction flap and stromal bed morphology assessment with anterior segment optical coherence tomography”, J Cataract Refract Surg, 38, 1544–1551 (2012). PMID: 22906441. T Seiler, F Hafezi, “Corneal cross-linking-induced stromal demarcation line”, Cornea, 25, 1057–1059 (2006). PMID: 17133053.
