By Ulrich Meyer-Bothling, Consultant Ophthalmic Surgeon, Ashford and St Peter’s Hospitals NHS Foundation Trust, and Ian Grierson, CCO of PolyPhotonix, Emeritus Professor of Ophthalmology, University of Liverpool, UK
PolyPhotonix, the manufacturer of the Noctura 400 – a treatment for diabetic retinopathy – recently completed a realworld study at an NHS hospital in the UK, which began in 2019. The study analyzed the effects of enhanced patient interaction with and use of the Noctura 400 sleep mask in a group of 26 diabetics displaying diabetic retinopathy (DR) and diabetic macular oedema (DMO).
The results of the study were remarkable: 94 percent of the patients who completed the study saw clinical improvement or stability. But the results are even more impressive when considering that the patients taking part in the study were a vulnerable population advised by the UK government and the NHS not to attend hospital for treatment during the COVID-19 pandemic.
The purpose of the study
The study aimed to find if patient compliance could be maintained and determine the anatomical and functional consequences of consistent mask wear. Throughout the whole study, outcomes were positive, with a high level of consistent patient use of the mask, above 74 percent up to and beyond one year. Even during the first COVID-19 lockdown in England, patients maintained a 65 percent nightly light mask compliance. Statistically significant improvements in maculopathy and visual acuity (VA) were maintained to the end of the study (see details below). Anatomical improvement or stability was recorded in all but one of the study eyes. Moroever, the investigation shows that, given appropriate interactions with patients who are self-treating in a home environment, a high level of patient compliance can be maintained – even with disruptions to the normal hospital clinic setup.
Mechanism of action
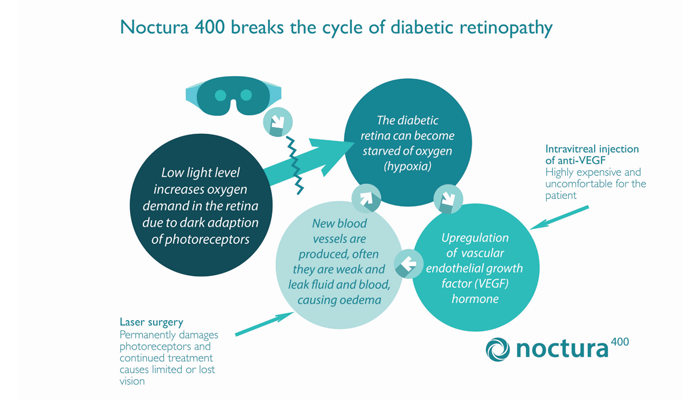
The Noctura 400 Sleep Mask prevents dark adaptation in the rod cells, which reduces the oxygen demand of the eye at night, reducing retinal hypoxia – a strain that not only affects choroidal circulation, but also compromises retinal vessels, potentially leading to DR and DMO.
The mask features an organic light emitting diode (OLED) housed inside a soft cushioned fabric mask – designed, of course, to be worn at night – that administers a precise dose of lowintensity light of a specific wavelength during a patient’s normal hours of sleep every night as part of a continuing therapy. Importantly, the mask also measures patient compliance; at the end of the allocated period, the mask is returned for analysis and a replacement is provided. Under normal circumstances collection and replacement happens in the clinic, but during pandemic-related disruption, new masks are posted to patients. The compliance data allows clinicians to compare how regularly the mask has been worn, which can then be matched with changes in vision and disease status.
Currently, DR is treated with laser and/ or injections of anti-VEGF pharmaceuticals directly into the eye. These treatments are expensive, demand substantial clinician time, and require patients to attend clinics on a regular basis. Visual healthcare and eye department budgets of many countries have been stretched by the success and rapid expansion of antiVEGF injection therapy for DMO. But it is also true that anti-VEGF injections and steroid implants are not without side effects and some level of patient distress. There is, therefore, a need for earlier treatment of limited-to-zero risk that should preferably be moved away from the clinic to the home – especially during COVID-19 outbreaks.
Study results
A total of 26 patients were recruited to the study; 24 (92.3 percent) reached their exit visit. Clinical assessment of DR and DMO, based on fundus examination and OCT, showed that in two patients both eyes improved; in 11 patients one eye improved while the other remained stable; 10 patients remained stable in both eyes; and in one patient one eye improved while the other developed proliferative diabetic retinopathy (PDR). Thus in 16/48 eyes (33.3 percent) the condition improved, in 31/48 eyes (64.6 percent) status remained the same at study end as at baseline, and only 1/48 eyes (2.1 percent) suffered deterioration.
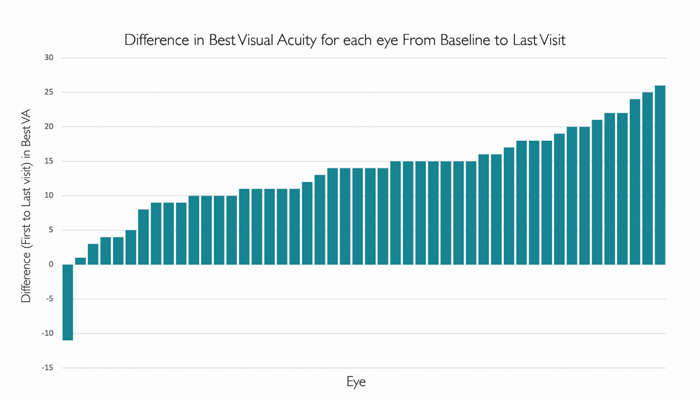
Notably, the majority of patients saw functional improvement based on VA. The waterfall plots (see Figure 1) show that almost all patients improved their acuity measures (VA improved in 47 eyes but deteriorated in one eye). Specifically, for 42/48 eyes (87.5 percent) the improvement was in excess of five letters; for 15/48 eyes (31.2 percent) the improvement was 15 letters or more. The mean and SD for improvement comparing baseline versus exit visit was highly significant by both paired t-test and signed rank with a mean best VA improvement of 12.4 +/-7.2 letters and a median of 14.
Maculopathy improvements were apparent after approximately six months. And on OCT examination, patients showed clinically significant anatomical improvements, such as a marked reduction in macular cysts (56.4 percent of eyes with cysts had clear cyst shrinkage), and the vast majority of patients exhibited anatomic stability or improvement during the study period, eliminating their need to engage with additional hospital treatments.
Today’s treatment of DMO is costly, invasive, and hospital based. The Noctura 400 is dramatically less expensive than current treatments and allows patients to take control of their treatment at home under the overall charge of their optometrists or hospital ophthalmologists. Noctura 400 is expected to profoundly change the future management of diabetic eye disease.