This article is an excerpt from a webinar with Optos® and LumiThera® , featuring Paulo E. Stanga (Consultant Ophthalmologist and Vitreoretinal Surgeon at the Retina Clinic London, London, UK), Marion Munk (Professor of Uveitis and Retinal Specialist at the University Hospital Bern, Switzerland), and Clark E. Tedford (President and CEO of LumiThera, Poulsbo, Washington, USA).
Please click here to view the on-demand webinar: “Shining a Light on Dry AMD (age-related macular degeneration): How photobiomodulation is helping dry AMD patients.”
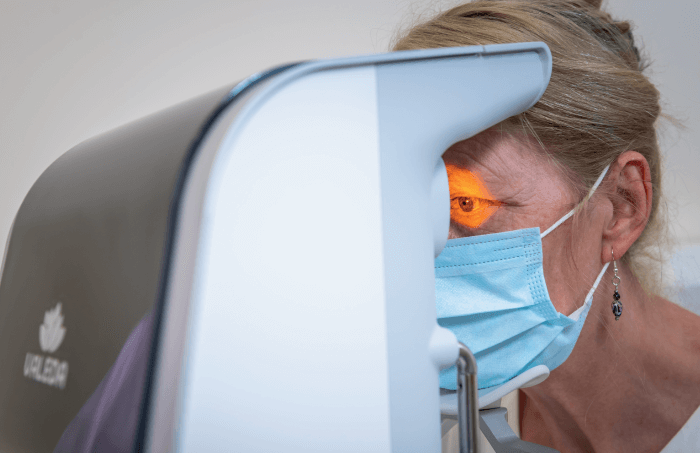
To understand the Valeda Light Delivery System, you must first understand the rationale behind the device. Dr. Clark Tedford (LumiThera, Inc.) explained how the technology came to be: “We wanted to develop a multiwavelength, non-invasive treatment to help improve visual function, stop or slow the progression of debilitating eye disease and, ideally, prevent vision loss and blindness.” To date, the Valeda is the first CE-approved treatment for dry AMD using photobiomodulation (PBM). PBM is a form of low-level light therapy that activates components of the mitochondria to stimulate a variety of cellular functions.
The beneficial effects of PBM are attributed to activity at cytochrome c oxidase – one of the five enzymes in the electron transport chain that is involved in driving bioenergetic outputs within the cell. Photobiomodulation improves blood flow, enhances oxygen binding, improves ATP formation, reduces oxidative stress, and overall improves cellular metabolic function. Valeda uses a light-emitting diode system with multiple wavelengths (590, 660, and 850 nanometers) to deliver a noncoherent, eye-safe column of light to treat the eye through either the open or closed eyelid. Each treatment takes less than five minutes with no need for pupil dilation. Patients are treated nine times over the course of three to four weeks to reset metabolic activity and provide extended benefit.
Marion Munk (Bern Imaging Center) shared the results of the LIGHTSITE I randomized, controlled trial. LIGHTSITE I enrolled 30 patients (46 eyes) randomized 1:1 into PBM or sham treatment groups. All participants in the study were diagnosed with dry AMD by fundus autofluorescence (FAF) and ocular coherence tomography (OCT) and had between 20/40 and 20/200 best- corrected visual acuity (BCVA). The study provided nine sessions over the course of three weeks, with a second treatment at six months. “After the first round of treatment, we saw an improvement in BCVA that maintained itself to about six months, where we started to see some remission. When patients came in for a second round of treatment, we observed restoration of improvement in BCVA,” explained Munk. Approximately 50 percent of participants gained between five and 22 letters’ gain on BCVA with some long-term remission, indicating repeat treatments are required to maintain improvement.
Alongside improved BCVA, patients also experienced improved contrast sensitivity, microperimetry, fixation stability, and visual function. It appeared that intermediate patients responded best to treatment, whereas the majority of low-responding patients (less than five- letters improvement) had a more advanced disease state. Munk concluded that PBM efficacy correlated to the different stages of dry AMD; earlier stages with no significant geographic atrophy showed better clinical and functional outcomes than patients with late-stage AMD.
Munk highlighted the morphological parameters of the trial, including drusen volume, fundus autofluorescence patterns, and the precursors of geographic atrophy growth: RPE and autoretina atrophy. Approximately 70 percent of patients in the PBM-treated group showed a reduction in drusen volume over the treatment period, whereas 100 percent of sham subjects showed an increase in drusen volume, which then resulted in significant drusen volume change from baseline to 12 months between groups.
With such promising clinical results, now there is hope for patients suffering from dry AMD. LumiThera is running multiple ongoing studies to further evaluate the clinical and anatomical outcomes of its treatment in dry AMD and other ocular diseases, with the ultimate goal of helping physicians address unmet patient needs.
www.optos.com
