- Visual field testing and OCT are not always sensitive to glaucomatous changes, delaying diagnosis of disease
- Pattern electroretinography (ERG) can detect subtle changes within the retinal ganglion cell (RGC) layer, sensing abnormalities before these cells atrophy and die.
- Pattern ERG data works well in synergy with other tests, such as visual evoked potentials (VEPs), which helps differentiate glaucoma-related optic neuropathy from other conditions
- Pattern ERG helps monitor therapeutic efficacy so that adjustments can be based on each patient’s disease features.
Current methods of glaucoma diagnosis using visual field (VF) testing or OCT can fall short on their sensitivity to glaucomatous changes, partly because of their dependence on surrogate markers of VF deficits. Even markers which accurately reflect VF loss only signal neuronal cell death – which remains irreversible. Far better would be to diagnose the disease at an earlier stage, when retinal cells are only damaged rather than already dead.
Pattern electroretinography (ERG) detects subtle changes in electrical responses within the retinal ganglion cell (RGC) layer, and is capable of sensing abnormalities well before these cells atrophy and die. As an objective measure of function, the test is much more sensitive to glaucomatous changes than either VF testing or OCT, and provides invaluable data to identify glaucoma suspects and inform the diagnosis and management of glaucoma patients.
The importance of interpretation
Pattern ERG testing has several potential uses when it comes to following a glaucoma suspect patient. But not all pattern ERG tests are equivalent; different modalities used for testing may provide rather different results. In particular, the slower stimulus frequency used in transient pattern ERG systems generates two response patterns: a positivity at ~50 ms (P50) and a larger negativity at ~95 ms (N95) (1), which reflect dysfunction in the macular and RGC regions, respectively. One challenge with transient pattern ERG results is that both P50 and N95 signals interact with signals from adjacent cells and neuronal generators, which can complicate the interpretation of whether pathology resides in the ganglion cell layer, other retinal layers, or structures within the visual pathway anterior to the lateral geniculate.
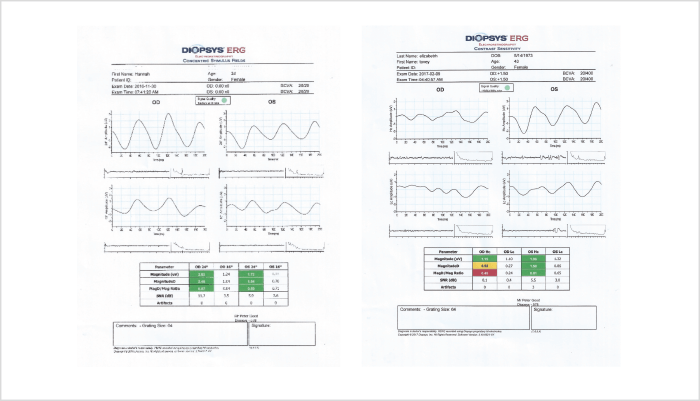
Pattern ERG testing with a steady state modality, like the testing offered from Diopsys platforms, can be much less ambiguous. The steady state modality increases metabolic demand within RGCs, and leads to functional habituation. Any delay in subsequent RGC recovery – changes in phase or amplitude – represents an objective indicator of ganglion cell dysfunction. Some instruments report these abnormalities with a flag system (Figure 1): a convenience which removes the need for subjective electrophysiological interpretation, and assures the clinician that the changes in RGC electrophysiology truly represent a loss of functionality in the cells that matter most in glaucoma.
Also, pattern ERG data can work in synergy with other electrophysiological tests – in particular, visual evoked potentials (VEPs). These measure the output of the central visual pathways, up to and including activity in the occipital cortex, and are especially useful when VF tests are inconclusive (as they often are). Measuring the VEPs evoked by a low-contrast stimulus will disclose glaucoma-related damage in the magnocellular pathway; by contrast, glaucoma-irrelevant pathologies affecting the parvocellular pathway are detected using a high-contrast stimulus. Thus, VEP testing helps differentiate glaucoma-related optic neuropathy from other conditions; after confirming the diagnosis in this way, pattern ERG can be used to assess severity and monitor progress.
Treatment decisions rarely hinge on a single piece of evidence. More often, good patient management requires consideration of data from many diagnostic modalities. However, different types of information usually have different – and additive – advantages in managing glaucoma patients. Below are three clinical scenarios that describe how I exploit the specific advantages of steady-state PERG in order to develop rational treatment decisions.
Scenario 1: Patient has elevated IOP but no other evidence of glaucoma
- Perform baseline test with steady-state PERG; if results are normal, repeat PERG every 6 months to monitor status
- If PERG remains normal after one year, and no other evidence of glaucoma is apparent, follow-up frequency may be reduced to two-year intervals
- If PERG remains normal after a total of three years follow-up, there is probably no mechanical explanation for the elevated IOP (i.e., angles are not narrow); conclude that the patient is unlikely to progress to glaucoma
Scenario 2: Patient has elevated IOP and abnormal baseline PERG, but no other evidence of glaucoma
- Repeat PERG after three months; initiate treatment if follow-up PERG data are abnormal
- If therapy is initiated, repeat PERG after one month; improved PERG readings will validate the initial indication of RGC dysfunction
- Repeat PERG every six months to one year, adjusting therapy or switching interventions as necessary
Scenario 3: Patient has elevated IOP, irregular OCT findings, VF defects and abnormal PERG readings
- Presentation suggests relatively advanced glaucoma which may require aggressive treatment to control IOP
- Use PERG measurements to: monitor response to therapy; titrate treatment accordingly; and/or choose from available treatment options (drops, SLT, stents or more aggressive drainage procedures).
Guiding decisions
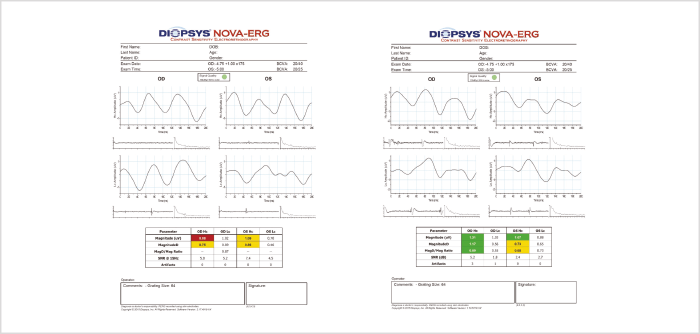
Numerous studies have shown that steady-state pattern ERG detects changes in the RGC layer several years before they are evident by OCT or visual field assessment (2). But are these very early loss-of-function findings clinically relevant? My experience is that they are: I have seen a number of patients in whom the steady-state pattern ERG signal improved after starting treatment (Figure 2), in the absence of documented evidence of structural changes per OCT, and this type of observation has been documented by others (3). The implication is that if patients’ pattern ERG can be stabilized, or at least kept within the normal range for that patient, then additional RGC loss can be avoided.
I believe it is feasible to base treatment decisions on pattern ERG data. Initiating treatment on the basis of pattern ERG data is especially justified if other clinical markers of glaucoma are present, such as IOP elevation, relevant family history, cupping of the optic disc, or progressive VF loss (see The Power of PERG: Three Scenarios).
A great benefit of the pattern ERG approach is that treatment may commence earlier than is possible with either OCT or VF data. Indeed, VF or OCT abnormalities are not unambiguous indicators of glaucoma, and clinicians who rely on these tests are faced with two unsatisfactory options: either initiate therapy and follow up to assess the eye over time, or monitor the patient to identify any changes that would unambiguously indicate that therapy should be initiated. The first option may expose patients to unnecessary treatment, and will result in health services incurring potentially avoidable costs; the second option risks irreversible vision loss in the patient. Pattern ERG, in contrast, enables clinicians to make better-informed treatment decisions much earlier in the disease process, such that prevention of vision loss is more feasible.
Consequently, pattern ERG not only triggers a decision to start treatment, but also helps avoid treating patients unnecessarily. For example, high myopia may induce abnormal OCT findings; in the absence of any other evidence of glaucoma, such patients would require imaging follow-up for two or more years to determine if these abnormalities were glaucoma-related. Pattern ERG, however, exposes RGC function and thus indicates whether structural abnormalities are indeed due to glaucoma, thereby avoiding the treat-or-monitor conundrum. Unnecessary treatment does not just expose patients to unnecessary risk, it also incurs unnecessary costs to the system; tailoring therapy to the individual patient avoids both, and pattern ERG is an excellent aid in this process.
Once patients have been diagnosed with glaucoma, following them with pattern ERG allows me to monitor therapeutic efficacy and to make adjustments based on each patient’s disease features. I believe pattern ERG is a more reliable marker of glaucoma progression than IOP, which does not correlate perfectly with either structural or functional loss and indeed is inconsequential in low tension glaucoma. Therefore, assessing the efficacy of IOP reduction therapy requires follow-up with other diagnostic modalities. As discussed above, it can take a long time for changes in disease status to become unambiguously manifest using standard tests (OCT or VF). Why wait so long for such important information when you can get the answer far more rapidly with pattern ERG?
Conclusions
Glaucoma is a complex clinical entity, affected by many variables. Diagnostic data must be interpreted in the context of other findings, which is why we take manifold diagnostic measurements in glaucoma cases: IOP readings, fundus examinations and so forth. Do we really need additional data points? In terms of pattern ERG, I believe the answer is unequivocally “yes.” I find the quantitative and qualitative information provided by steady-state pattern ERG extremely helpful, both in its own right and in making sense of other clinical findings. Crucially, basing treatment decisions on pattern ERG findings may prevent RGC death, and therefore avoid VF loss. For example, pattern ERG may help determine if current IOP is sufficient to stabilize disease, or if additional measures should be taken (for example, if abnormalities persist or worsen). Such decisions can be made without pattern ERG – but why do without additional information that gives greater clarity and more confidence in glaucoma management?
Peter Good is the head of the Visual Function department at Birmingham Midland Eye Centre, Birmingham, UK.
References
- GE Holder, “Pattern electroretinography (pattern ERG) and an integrated approach to visual pathway diagnosis”, Prog Retin Eye Res., 20, 531-61 (2001). PMID: 11390258. MR Banitt, et al., “Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects”, Invest Ophthalmol Vis Sci, 54, 2346-52 (2013). PMID: 23412088. LM Ventura and V Porciatti, “Restoration of Retinal Ganglion Cell Function in early Glaucoma after Intraocular Pressure Reduction”, Graefes Arch Clin Exp Ophthalmol, 247, 1223-1233 (2009).PMID: 15629815.
