- For the first time, aqueous angiography has been applied to living subjects (both humans and non-human primates)
- Real-time data from live patients was consistent with previous post-mortem aqueous angiography: outflow is segmentally heterogeneous
- Furthermore, live-patient data confirmed a pulsatility to outflow and resulted in the discovery of dynamic features of aqueous outflow – a unique observation
- Increasingly, aqueous angiography appears to have the potential to guide surgery to patient-specific regions, thereby enhancing MIGS outcomes.
Impaired aqueous humor outflow (AHO) is usually associated with resistance in the trabecular outflow pathways – the trabecular meshwork (TM), Schlemm’s canal (SC) and collector channels (CC). So it makes sense (on the face of it) that procedures aiming to bypass or ablate the TM – minimally-invasive glaucoma surgery (MIGS) – are increasingly popular. Big question then: why don’t trabecularly-oriented MIGS procedures drop IOP dramatically in every patient? At least part of the problem may be that AHO is not uniform around the limbal circumference. As some segments have better outflow than others, it’s almost certain that some are better sites for a MIGS procedure than others. Clearly, we need a tool that allows detailed visualization of the AHO idiosyncrasies in each individual patient, helping us identify sites of outflow resistance. Such a tool would take the guesswork out of the MIGS game, and might permit truly personalized glaucoma surgery. But what would be the key features of such a tool? The ideal tool would be able to provide real-time, physiologically-relevant and comprehensive information from the patient’s eye in situ. In this context, ‘comprehensive information’ would cover structure and function across all trabecular AHO pathways in their entirety: both linearly (from the anterior chamber [AC] to the episcleral vein) and circumferentially (360° of coverage). But how close are we to this ideal?
Assessing AHO
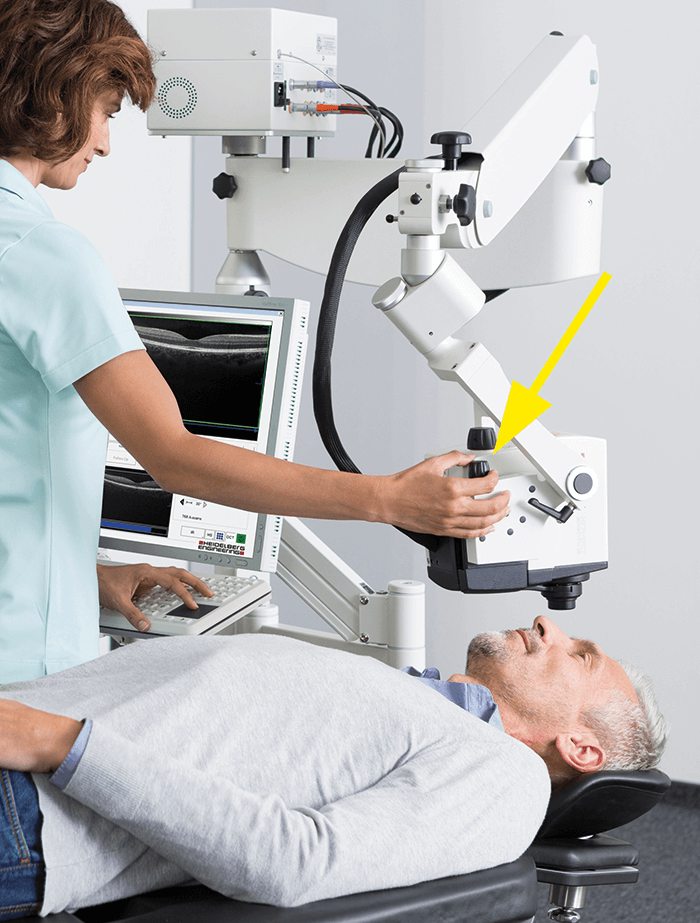
We’ve had a tool for the non-invasive structural assessment of AHO architecture in living individuals for some time. Anterior segment OCT (AS-OCT) can image AHO structures; however, the resolution provided by the typical/commercial B scan–B scan distance of ~35 microns) is too coarse to pick up many collector channels, and the number of OCT ‘slices’ required to collect a full representation of the AHO in a given eye is a challenge. That said, variations on the technique have provided useful insights into AHO biology: phase-based OCT has demonstrated pulsatile AHO flows in live human eyes. Nevertheless, AS-OCT is not equivalent to true functional studies because it doesn’t tell us anything about the relationship of structural variations to functional differences in AHO. For example, does an unusually large CC lumen always indicate very active flow versus a cul-de-sac filled with stagnant fluid? To start, it may be preferable to have a technique that provides true functional assessments of the AHO, which is to say visualization of fluid flow. Previous efforts in this field have included injection of particulate tracers – such as nanoparticles or fluorescent microspheres – into the AC, followed by microscopy of sections of the eye. Of course, this approach is not compatible with live patients. Fortunately, other techniques may permit at least a degree of real-time, functional imaging from intact eyes: episcleral venous waves (described by Ron Fellman, Glaucoma Associates of Texas), canalography (where tracer is introduced into SC) and aqueous angiography (where tracer is introduced into the AC).Aqueous angiography is the newest functional AHO imaging approach. In our lab, we have developed an ever-refining method that uses indocyanine green (ICG) and/or fluorescein tracers to visualize fluid flow, with images being captured by a Spectralis HRA+OCT (Heidelberg Engineering). It’s fair to say that methods development required some out-of-the-box thinking in the early days; for example, our Spectralis HRA+OCT instrument was designed for patients with chins, so it wasn’t immediately applicable to the post-mortem pigs and cows we used for our initial studies... The solution? We obtained styrofoam heads from a cosmetics school and placed enucleated eyes into drilled holes. Subsequently, to image in the operating room, the Spectralis FLEX module was developed (Figure 1). These tools allowed us to develop a robust method (See Box, Aqueous angiography method outline), which has yielded encouraging data in a range of settings (post-mortem pig, cow and human eyes; live non-human primates (NHPs); and live humans).
Validating the past, discovering the future
Our initial experiments indicated that aqueous angiography was a valid means of visualizing AHO; in particular, multi-modal imaging confirmed that the angiographic signal corresponded to AHO structures (for example, AS-OCT showed that intrascleral vessel lumens overlapped with angiographically-positive vessels identified by aqueous angiography; and in laboratory experiments, tracers accumulated preferentially in the TM of angiographically-positive regions). Furthermore, segmental variation was seen in all species, confirming that AHO vessel distribution is non-uniform around the circumference. In sum, these data suggested that aqueous angiography could be a useful technique to answer fundamental questions regarding AHO function in diseased and healthy eyes. The ideal location for trabecular MIGS was one of the first questions we addressed. Should the surgery focus on a low-flow region (because in high-flow regions, the TM may be offering little resistance; therefore, bypassing the TM would be of little benefit); or should the surgeon avoid low-flow areas (because they may be intrinsically poor drainage sites, because of anatomy, for example). Investigating the issue required a two-tracer system to be devised. Briefly, the native state of the eye is first investigated by ICG-based aqueous angiography; subsequently, the effects of trabecular bypass stents are gauged using fluorescein-based aqueous angiography. Using this two-tracer technique, we generated data from post-mortem cow eyes and enucleated human eyes that strongly suggested regions of low flow could be rescued by trabecular bypass surgery (1). Nevertheless, a definitive answer to the question required data from subjects that would be better models for actual human patients. Aqueous angiography had never before been used in living subjects, and we found that its application in NHPs and humans required yet more inventiveness. An immediate problem was raised by the Spectralis HRA+OCT design – it is intended for upright patients, but in the operating room, patients are supine. To address this, we modified the system by mounting it on a modified surgical boom arm with multi-pivot joints (Spectralis FLEX module).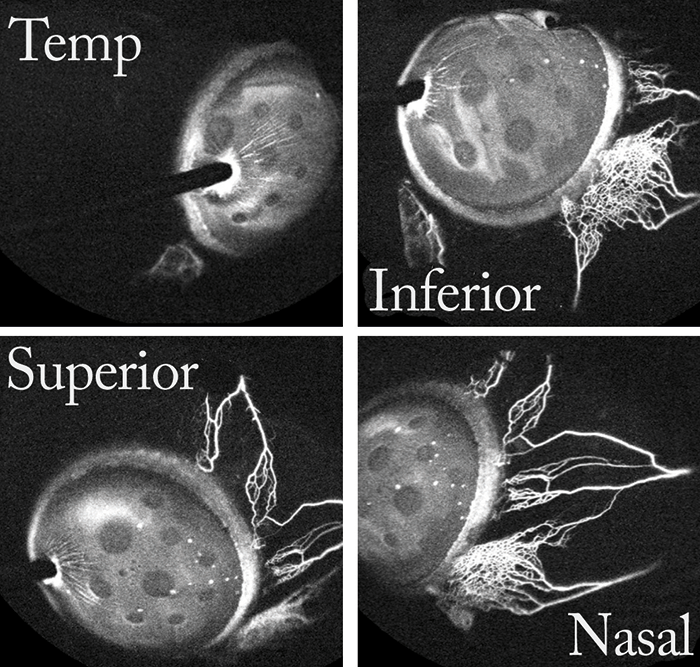
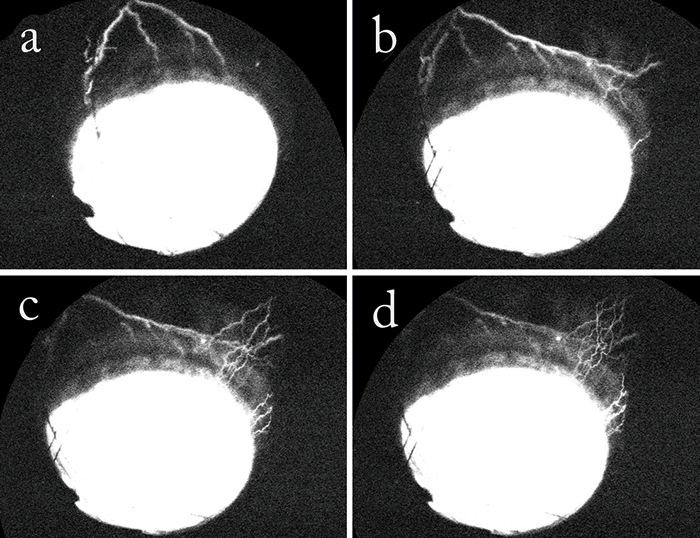
When we applied our method to NHPs with Ningli Wang’s lab at Tongren Hospital, in Beijing, it was the first ever attempt to use aqueous angiography in living subjects (2). Gratifyingly, data generated from living primates confirmed our earlier findings from post-mortem subjects regarding the segmental (circumferentially heterogeneous) nature of AHO. Similarly, findings from live NHPs also confirmed the pulsatile nature of AHO. Interestingly, the pulsatile flow was evident despite the use of a constant pressure system to effect tracer delivery. Other groups, such as Murray Johnstone from University of Washington, have suggested the pulses are of cardiac origin. We did not specifically investigate this, but we noted that the NHP pulsation rates we observed by aqueous angiography were similar to published average NHP heart rates. Even more excitingly, we can now report similar data from live humans (3) done with Robert Weinreb at University of California, San Diego. Briefly, aqueous angiography images, using ICG tracer, were taken from eight patients during phacoemulsification. Again, segmentally heterogeneous and pulsatile AHO characteristics were observed (Figure 2). More interesting still, however, was our observation – seen both in NHPs and in human patients – of a dynamic aspect of AHO. It was an entirely novel observation, and was manifest both as the growth of active flow in regions that previously did not have an angiographic signal, and as the diminishment of flow in regions with a strong initial angiographic signal (Figure 3). The mechanism behind these fluctuations remains unclear. In summary, these studies demonstrate that aqueous angiography is possible in the eyes of living human subjects, that it is compatible with successful and complication-free phacoemulsification, and that there is a hitherto unsuspected dynamic element to AHO.
- Anterior chamber maintainers are used in preference to standard needles, as their grooved ridges result in less sliding and less leakage at the entry point.
- Constant pressure, gravity-driven tracer delivery is effected by means of a reservoir positioned above the eye.
- Pressures are set at 10 mmHg (enucleated eyes) or 20 mmHg (intact eyes of living subjects).
- Spectralis HRA+OCT system is mounted on a modified surgical boom arm with multi-pivot joints to permit multi-positional imaging of supine primate subjects.
- After establishing a dark pre-tracer background, images are captured with the angiographic function, in either fluorescein capture or ICG capture mode.
- For living subjects, a lid speculum is required. Also, note that eyelids block the post-limbal view in living subjects: for non-human primates, use traction sutures to rotate the eye, and for humans, instruct patients to move eyes as necessary.
What’s in the AHO pipeline?
The dynamic aspect of AHO deserves further investigation; establishing the biological mechanism behind altered flow in a given area may point to new ways of pharmacologically or surgically modulating outflow. Furthermore, AHO detection in live patients would allow identification of differences between diseased and normal eyes, and may lead to the answer regarding surgical choice by identifying optimal sites for surgery. Potentially, this might not only improve the predictability of trabecular MIGS procedures, but also increase the magnitude of IOP improvement provided by these interventions. One can also envisage surgeons and scientists learning from both canalograms and aqueous angiograms in a given eye. Since the AHO contribution of TM is equivalent to the canalography result minus the aqueous angiography result, a comparison of the two measurements would allow the surgeon to distinguish between resistance contributions of proximal AHO pathways (which is to say, TM) and distal AHO pathways (post-TM). Access to such a comparison may also have implications on clinical decision-making. However, if aqueous angiography is to take its rightful place in the ophthalmologist’s toolkit, further refinement of the method is required. At present, tracer delivery is invasive and in the AC, as opposed to the sulcus where aqueous normally arises; in addition, the use of a lid speculum when imaging live subjects may alter ocular surface pressure, and antimuscarinic dilation drops (used in subjects undergoing cataract surgery) may change TM capacity. These factors could, in theory, contribute to angiographic artifacts and will need to be addressed in future iterations of aqueous angiography. Nevertheless, we believe the technique will soon be used to compare AHO in normal and glaucomatous eyes (although careful attention will be required to exclude patients with low pressure glaucoma). In the longer-term, we hope that the method will be made less invasive. For true non-invasive imaging, it will be required to identify a marker present at much higher levels in aqueous humor than in serum, such that AHO can be distinguished from ocular blood flow without the need for an externally administered tracer agent. In this context, it is exciting that vitamin C is present at 100-fold higher concentrations in aqueous humor as compared with serum; unfortunately, its fluorescence characteristics are not compatible with current clinical imaging technologies – but who knows what the future holds? Alex Huang is Assistant Professor at the Doheny and Stein Eye Institutes, Department of Ophthalmology, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA. Huang is a glaucoma specialist and advanced cataract surgeon who supports all current and minimally invasive glaucoma surgical procedures. Huang carries his interests regarding angle-based approaches and native outflow pathway improvement into the laboratory as a National Institutes of Health-supported clinician-scientist. His lab explores post-trabecular meshwork outflow resistance as well as real-time aqueous outflow imaging technologies for the development of customized glaucoma surgeries. Huang’s clinical practice emphasizes a balance of modern surgical techniques with traditional approaches to ensure optimal glaucoma management. In 2017, Huang was voted #1 on The Ophthalmologist Rising Stars Power List.References
- AS Huang et al, “Aqueous angiography-mediated guidance of trabecular bypass improves angiographic outflow in human enucleated eyes”, Invest Ophthalmol Vis Sci, 57, 4558–4565 (2016). PMID: 27588614. AS Huang et al, “Aqueous angiography in living non-human primates shows segmental, pulsatile and dynamic angiographic aqueous humor outflow”, Ophthalmology, 124, 793–803 (2017). PMID: 28237425. AS Huang et al, “Aqueous angiography: aqueous humor outflow imaging in live human subjects”, Ophthalmology, 124, 1249–1251 (2017). PMID: 28461013.
