Diabetic retinopathy (DR) is one of the most common causes of blindness in the world – yet most of this sight loss is preventable, if treatment is given early. Unfortunately, the majority of currently available treatments are prohibitively expensive, highly invasive, and present a significant burden to patients and healthcare systems, leaving many patients untreated.
The retina uses more oxygen per unit mass than any other tissue in the body, thanks to the phenomenally high metabolic rate of photoreceptor cells. The high demand for oxygen becomes even greater at night, rising by around 40 percent, as rod photoreceptors dark-adapt. Under normal circumstances this isn’t a problem; additional oxygen demand is met by increased retinal vasculature blood flow. It’s no surprise that problems start to occur when the vasculature becomes damaged – a common feature of diseases, such as DR and diabetic macular edema (DME).
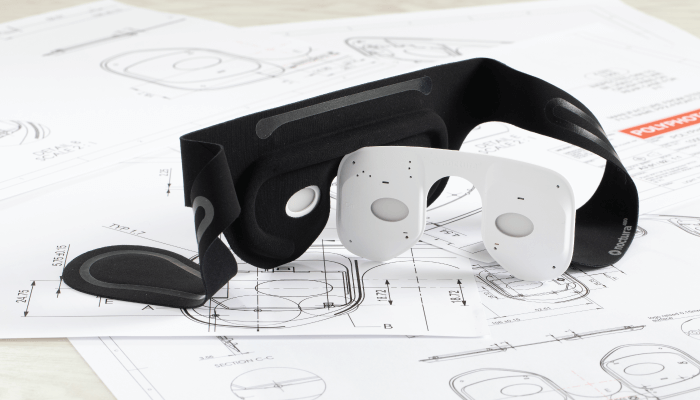
Noctura 400 is a home-use, clinician prescribed (and monitored) treatment for both early and later-stages of the condition. It can also be used, in conjunction with good glycemic control, to help prevent early onset. And it seems more relevant now than ever; after all, COVID-19 has shone a new spotlight on the need for effective home-based treatments – especially for high-risk patients, including those with diabetes (the second most common underlying condition among COVID-19 patients who have died from the disease). Worn at night during sleep, Noctura 400 treatment prevents the rods from dark adaptation, which reduces the oxygen demand of the eye at night, thus preventing hypoxia and the consequent compromised blood vessel proliferation that can result in the retina.
The Noctura 400 mask registers patient usage time and this information is uploaded to a PolyPhotonix-designed software system (PPX Works), which can be accessed by healthcare providers and treatment payers. Monitoring can be done remotely and the collected compliance data shows how regularly the mask has been worn – this data that can be matched with changes in visual acuity and the overall condition of the disease.
Noctura 400 already has CE marking in Europe and regulatory approval for use in a number of countries around the world.
The most recent real-world study on Noctura 400, carried out by the NHS on a group of 26 patients with diabetes displaying DR and DME, showed clinical improvement or stability in 94 percent of the patients who completed the study.
Noctura 400 revolutionizes DR treatment at a very low cost – with the aim of providing affordable treatment to the millions of people affected around the globe.
PolyPhotonix is the manufacturer of Noctura 400.
