In the fight against neovascular age-related macular degeneration (AMD), there are three heavyweights in the arena: ranibizumab, aflibercept, and bevacizumab. Although bevacizumab is commonly used off-label, ranibizumab and aflibercept are both indicated for the treatment of neovascular AMD. But which to choose?
It’s known that all three are roughly similar in terms of efficacy and safety – aflibercept’s approval was based on the Phase III VIEW trials which found that it was non-inferior to ranibizumab (1),(2), and the CATT trial showed comparable outcomes with both ranibizumab and bevacizumab (3). But we also know that the real-world outcomes of patients receiving ranibizumab for the treatment of neovascular AMD don’t match those in the clinical trials (4). So across all of the “heavyweights,” is there a best choice amongst them in the real world?
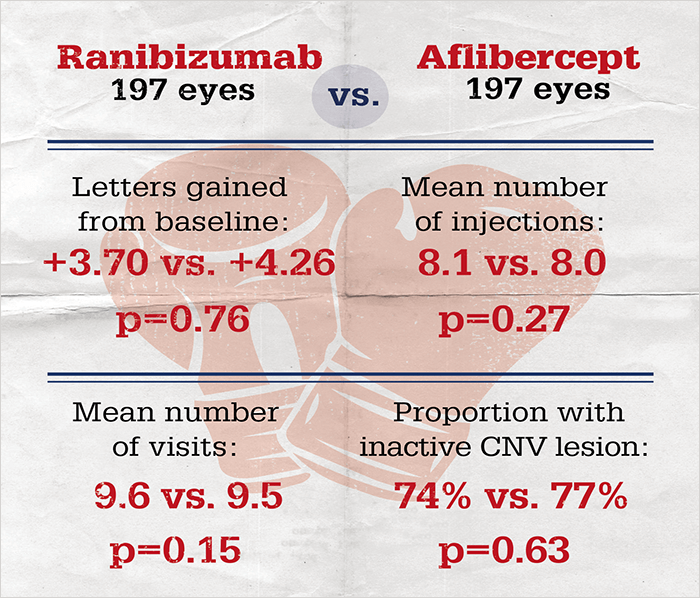
To address this question, a multinational team mined the Fight Retinal Blindness registry to directly compare outcomes of patients treated with ranibizumab versus those treated with aflibercept (5). What they found was that patients’ outcomes were similar after 12 months of treatment, irrespective of the drug used: there were no significant differences in visual acuity (VA) improvement nor frequency of treatment between eyes treated with either drug (Figure 1). They also found that more patients switched from ranibizumab to aflibercept than aflibercept to ranibizumab (13.7 percent vs. 3 percent, respectively), but that there was no VA benefit associated with the switch. Concluding that both drugs “delivered similar, good outcomes in routine clinical practice,” the authors acknowledge that “a randomized controlled trial would be required to demonstrate the superiority of one drug formally; however, numbers would be prohibitively large based on event estimates from this study.”
References
- U Schmidt-Erfurth et al.,“Intravitreal aflibercept injection for newovascular age-related macular degeneration: ninety-six-week results of the VIEW studies”, Ophthalmol, 121, 193–201 (2014). PMID: 24084500. JS Heier et al., “Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration”, Ophthalmol, 119, 2537–2548 (2012). PMID: 23084240. DF Martin et al., “Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results”, Ophthalmol, 119, 1388–1398 (2012). PMID: 22555112. FG Holz et al., “Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration”, Br J Ophthalmol, 99, 220–226 (2014). PMID: 25193672. MC Gillies et al., “Twelve month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: data from an observational study”, Ophthalmol, 123, 2545–2553 (2016). PMID: 27707549.
