You might be forgiven for thinking that with all the advances in retinal stem cell science, researchers are able to perform their experiments on any of the major retinal cell types – and with animal-derived cell stem cell lines, that’s fair description of where the science is. That’s also mostly true with human pluripotent stem cell (hPSC) cell lines – apart from retinal ganglion cells (RGCs). hPSC RGCs have been successfully produced, but those produced by current methods are challenging to purify in large numbers, express a relatively small number of RGC-associated genes, and exhibit “limited physiological characterization of the derived cells” (1), (2), (3), (4).
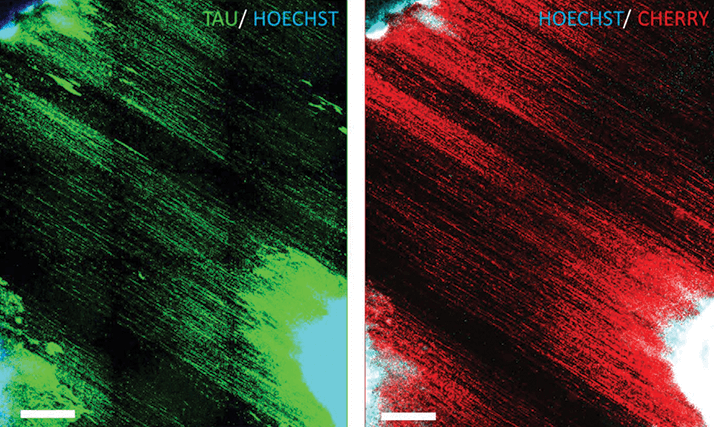
Now, a team of Maryland-based researchers from the Johns Hopkins University School of Medicine and the National Institute of Neurological Disorders and Stroke, in Baltimore and Bethesda, respectively, have described a simple adherent cell protocol for growing and differentiating human embryonic stem cells into RGCs (1). They used the CRISPR-Cas9 gene editing technique to place mCherry, a red fluorescent protein reporter gene, into the stem cells’ DNA within the open reading frame of the BRN3B gene, an important and well-characterized marker of RGCs. What this means that if the cells fluoresce bright red under 587 nm wavelength illuminations, they’re highly likely to be RGCs. The fact that the stem cell culturing protocol is adherent means that the cells will grow and grow until the entire surface of their container is covered – something that enables large volumes of cells to be cultured. The fact that the RGCs can glow red under the right illumination means that they can be isolated by a fluorescence-activated cell sorting (FACS) machine – leaving researchers with copious amounts of highly enriched RGCs, which on inspection, the researchers found that the cells expressed functional glutamate receptors and could fire spontaneous and induced action potentials (like RGCs should) – making this an immensely useful tool for retina researchers. While seeking to optimize the process, the research team discovered new ways to finesse their technique. The first: adding forskolin early in the culture process, which increased the proportion of RGC cells in the culture medium. The second, providing a nanofiber matrix (that mimicked the highly aligned nature of the optic nerve in vitro) guided the RGCs to develop aligned axons (Figure 1) that resembled RGC axons in the retinal nerve fiber layer and the optic nerve. Even better, the study authors believe that the use of CRISPR-based gene editing to make reporter lines could be used to introduce mutations associated with optic nerve disease, and enable the “direct comparison of pertinent mutant versus wild-type cells and thereby aid greatly in efforts to elucidate novel disease mechanisms and develop potential therapeutics.”
References
- VM Sluch et al., “Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line”, Sci Rep, 5, 16595 (2015).PMID: 26563826. X Zhong et al. “Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs”, Nature Communications, 5, 4047 (2014). PMID: 24915161. JS Meyer et al. “Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment” Stem Cells, 29, 1206–1218 (2011). PMID: 21678528. T Nakano et al. “Self-formation of optic cups and storable stratified neural retina from human ESCs”, Cell Stem Cell, 10, 771–785 (2012). PMID: 22704518.
