Fusing the latest technology with human biology to bring sight to the blind, retinal prostheses capture the imagination. And though multiple devices are now on the market, they’re still not perfect. Complicated to make and challenging to implant, some require an external camera to function. Most require trans-ocular cables for power or data transfer (which risk damaging the soft tissue of the retina every time the eye moves) and there have been concerns about the longevity of such electrical components placed in distinctly wet biological tissues. Finally, patients achieve visual acuities of less than 20/200, so they are still legally blind. Can things be improved?
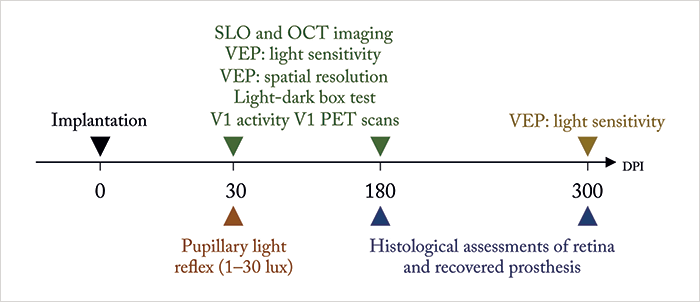
Maya-Vetencourt et al. (1) report the development of a fully organic retinal prosthesis that might fit the bill. Metal and silicon-free, it’s composed of a passive, silk fibroin substrate, a central conductive layer of poly (3,4-ethylene-dioxythiopene)-poly(styrenesulfonate) – PEDOT:PSS for short, and a superficial semiconductive layer of poly(3-hexylthiophene) – P3HT. In the past (2), it’s been shown that glass slides coated with P3HT can photoactivate neurons grown on the slide, and when implanted into rats with light-induced photoreceptor degeneration, it can restore light sensitivity – all without requiring a power supply or any external components. This flexible silk-based iteration gets rid of the glass – and possibly the soft tissue damage and degradation issues of current retinal prosthesis designs. They turned to Royal College of Surgeons (RCS) rats, a widely recognized model of retinitis pigmentosa, to test the design – and their experimental approach and assessments are depicted in Figure 1.
At 30 days post implantation (DPI), they found that, at illuminances of ≥4 lux, their prosthesis rescued the pupillary reflex, and that prosthesis-induced visually evoked potentials (VEPs) could be detected in the primary visual (V1) cortex in a manner that topographically represented the light-dependent activation of the inner retina circuitry harboring the prosthesis. A number of light stimulation paradigms paired with V1 VEP measurements were performed to assess the recovery of visual cortical response and spatial resolution, and visually-driven behavior was assessed using the light-dark box test. The organic retinal prosthesis performed significantly better than sham- and non-implanted RCS rat controls – and the prosthesis-dependent recovery of visual function persisted up to 6–10 months after surgery (when the rats were sacrificed for histological analysis). Further, positron emission tomography neuroimaging showed that the prosthesis’ rescue of visual function was associated with increased basal activity in V1 cortex. Clearly it’s working, but the question is how? The authors don’t actually know: “the detailed principle of operation of the prosthesis remains uncertain.”
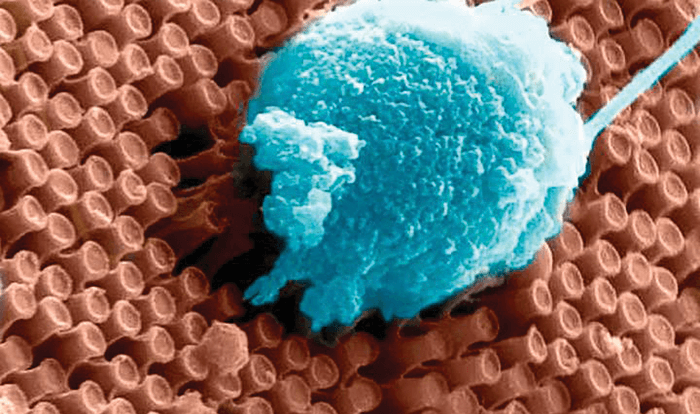
Meanwhile, engineers at the University of California San Diego and the La Jolla-based startup, Nanovision Biosciences Inc. have taken a different approach: optoelectronic silicon nanowires (Figure 2) that can both sense light and stimulate the retina (3). They’re powered by a wireless inductive system that transfers energy with up to 90 percent efficiency, and also allows both data transfer and control over stimulation paradigms. One of the engineers involved in the project, Gert Cauwenberghs, noted, “To restore functional vision, it is critical that the neural interface matches the resolution and sensitivity of the human retina.” They might be on the right track – the tiny size of the nanowires more closely match the dense spacing of photoreceptors in the human retina than the 60 and 1500 electrodes present in the Second Sight Argus II and Retina Implant Alpha IMP prostheses, respectively. But can it work? To provide proof of concept, the researchers performed in vitro electrophysiological experiments on the retinae from rhodopsin P23H knock-in rats (a model of retinitis pigmentosa-like retinal degeneration). And they were encouraged by what they found: the horizontal and bipolar neurons in the retina fired action potentials preferentially when the prosthesis was exposed to a combination of light and electrical potential – and were silent when either light or electrical bias was absent. In other words, they showed that the nanowire array successfully responds to light and electrical stimulation. Animal tests with the device are in progress, with clinical trials set to follow.
References
- F Maya-Vetencourt et al., “A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness”, Nat Mater, [Epub ahead of print] (2017). PMID: 28250420. D Ghezzi et al., “A hybrid bioorganic interface for neuronal photoactivation”, Nat Commun, 25, 166 (2011). PMID: 21266966. S Ha et al., “Towards high-resolution retinal prostheses with direct optical addressing and inductive telemetry”, J Neural Eng, 13, 056008 (2016). PMID: 27529371.
