Eye injuries that damage the limbus may result in limbal stem cell deficiency (LSCD) and failure of corneal regeneration. Stem cell transfer procedures can be curative, but have been hindered by the scarcity and immunogenicity of allograft material. Research indicates that allografts can trigger repopulation of the host stem cell niche with autologous cells (1), and this in turn suggests that cadaver limbus could be used as a universally-applicable graft material, in virtually unlimited quantities. Could cadaveric cells eliminate the need for autografts and ‘living donor’ allografts? Limbal stem cells are key to regeneration of the cornea after injury… but if the limbus also is damaged, natural corneal recovery may be compromised by LSCD, resulting in problems including dry eye, chronic inflammation, and loss of vision. Hence, external insults, such as physical, chemical or thermal injury, or diseases such as cancer, chronic limbitis or Stevens-Johnson syndrome, may lead to chronic visual defects.
The clinical treatment options for LSCD-based problems are limited, as traditional corneal graft procedures prove ineffective, and penetrating keratoplasty (PK) is contraindicated in these patients. Stem cell transplantation remains the best approach for severe cases, and various options are available to the surgeon according to tissue type and tissue provenance (Table 1).
To immunosuppress, or not?
Clearly, the only immunologically perfect match for the damaged eye will be autograft tissue (that is, cells taken from the contralateral eye). Historically, this has been the favored approach, as it avoids the need for long-term immunosuppression to avoid graft rejection. The autograft technique, however, is limited to cases where only one eye is damaged and where the patient is willing for the undamaged eye to be used as a stem cell source. And given that the procedure may involve removing two grafts of 90° of limbal arc, there is a small chance that the patient’s only healthy eye may be damaged. Understandably, some patients are reluctant to take that risk. The next-best source, from an immunological perspective, is allograft from a relative of the patient – the ‘living-related’ allograft. Again, the quantity of tissue is limited by the amount of harvesting a healthy eye can sustain. Furthermore, this procedure is only possible where relatives are alive, available and willing to donate corneal tissue. Finally, tissue may be sourced from non-related individuals, either living or cadaveric. Living non-related donors, however, have the same restrictions as living-related donors with regard to availability, risk to the donor eye and willingness to provide ocular tissue. Cadaveric donors, by contrast, are far less restricted in terms of availability and quantities of tissue that can be harvested per eye. Indeed, in vitro culture of the cadaveric corneal disc followed by multiple explants – each of which is used as a source of cells to expand in culture (Figure 1) – can provide sufficient cells for multiple procedures from a single eye.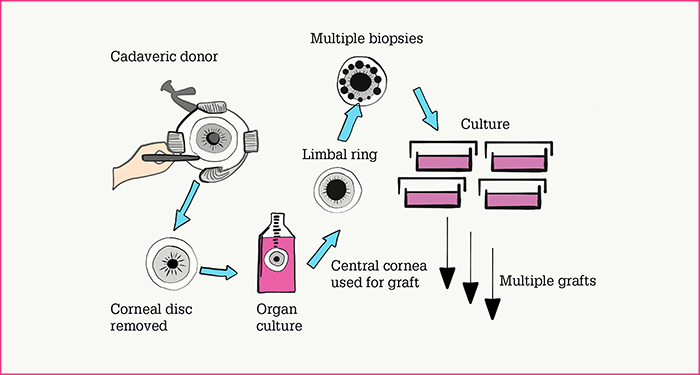
So, cadaveric eyes may provide an abundant source of tissue for limbal stem cell transplants and may be especially suitable for patients with bilateral LSCD – or those with unilateral damage who cannot access stem cells from living donors or those who prefer not to undergo an autograft procedure. The problem has always been that cadaveric allograft transplant recipients must suffer a regimen of long-term, systemic immunosuppression if the graft is to survive. Data from our laboratory, however, now challenge this orthodoxy.
Revenant cells
Briefly, our method is as follows.- Tissue sourcing. We remove the entire corneal disc from the cadaver eye, and maintain it in organ culture medium. When appropriate, we take multiple biopsies from the limbal ring and retrieve viable cells from each.
- Cell population expansion and harvesting. We expand the retrieved cells from each biopsy in separate cultures; this provides material sufficient for multiple graft procedures (Figure 1). Over time, small discrete colonies grow and merge into multilayered epithelial sheets; these are released from the plastic substrate with Dispase II enzyme (2).
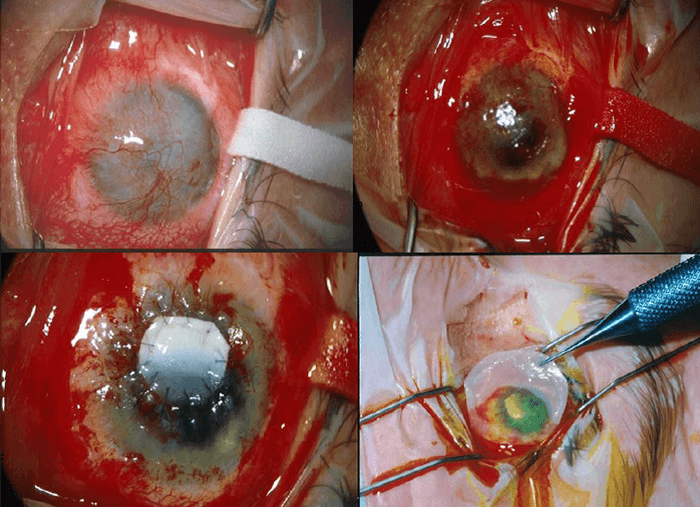
- Graft construction and implantation. The cultured epithelial sheet is mounted on a Tegapore dressing, which is used to transport the cultured sheet. The sheet is transferred onto the prepared ocular surface and then covered with amniotic membrane which is sutured to the bulbal conjunctival surface (Figure 2).
- Post-operative regimen. Our patients receive intravenous methylprednisolone (1–2 mg/kg/day) until inflammation in the eye has calmed down, plus cyclosporin A (2–3 mg/kg/day); and dexamethasone (0.1%), chloramphenicol and autologous plasma (each, qid).
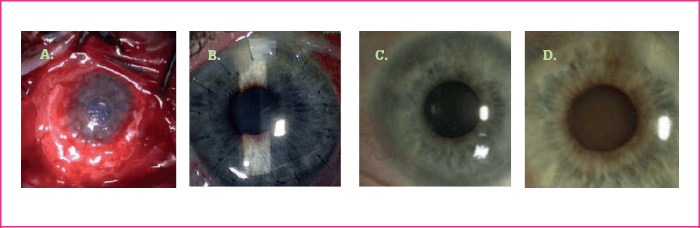
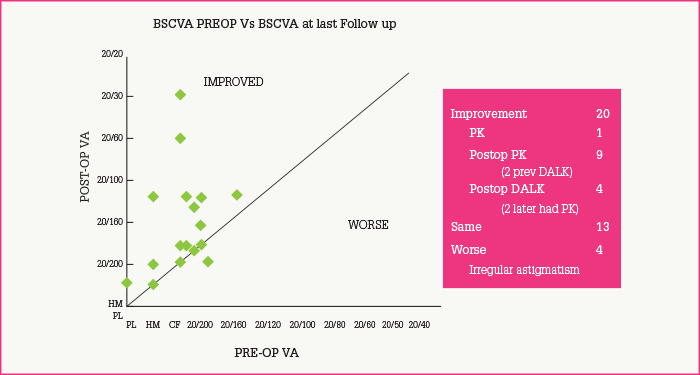
The above technique produces grafts that appear similar to the limbus by microscopy and molecular marker studies. Thus, the epithelial sheet forms multiple layers with a distinct basement membrane, and markers K19, K3 and enolase are appropriately distributed in the layers of the graft. Recipients have a number of variables, and the procedure is not perfect: there is a 29 percent failure rate, patients with conjunctival inflammation and lid problems being particularly prone to problems. When successful, ocular surfaces remain stable for periods of nine years or more (Figure 3a-d). Furthermore, the procedure results in marked improvement of symptoms, including decreases in inflammation, conjunctivitis, pigment epithelial detachment, vascularization, photophobia and pain (Figure 4), and improved visual acuity (Figure 5).
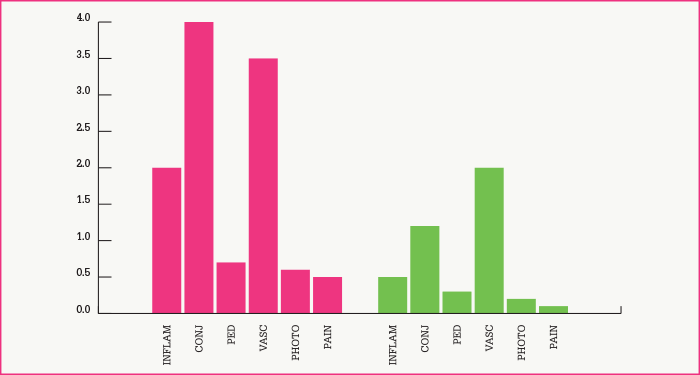
Perhaps the most important finding from our work, however, is that allografts not only can be curative in cases of LSCD, but also that they appear to do so by a mechanism involving regeneration of the host limbal stem cell population. The evidence for this is that donor cells do not populate the host limbus – indeed, our DNA analysis shows that they are absent from the host eye by nine months after implantation (1). More recently, others have reported similar findings in keratolimbal allografts (EJ Holland, personal communication). Our conclusion is that the host cells take over stem cell-mediated repair of the eye within a year of implantation, and that the grafted cells are lost during this period.
The death of living donors?
The implications of the findings are immense. Firstly, within ophthalmology, it suggests that there may no longer be a need for autograft or living allograft methods. Indeed, if long-term immunosuppression is no longer required for cadaveric allografts, then living donor methods are left only with comparative disadvantages regarding their availability, the risk to healthy eyes, the discomfort and inconvenience suffered by donors, and the quantity of cells that can be generated from a given eye. Cadaveric eyes have none of these disadvantages. Secondly – and more broadly – the idea that an unrelated allograft can trigger repopulation of a niche with autologous (host) stem cells is intriguing. Are totipotent or pluripotent stem cells being attracted from elsewhere in the body, such as the bone marrow, and then being triggered to differentiate into limbal stem cells? Or is there a transdifferentiation mechanism at work? Further elucidation of the pathways behind this fascinating observation could have massive implications for repair and regeneration of all types of tissue throughout the body. Sheraz Daya is the Founder and Medical Director of the Centre for Sight. He was amongst the first in the UK to perform LASIK, and he has pioneered a number of corneal and anterior segment techniques and invented several ophthalmic instruments. The author reports no relevant disclosures.References
- SM Daya et al., “Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction”, Ophthalmology, 112, 470–477 (2005). PMID: 15745776. G Pellegrini et al., “Long-term restoration of damaged corneal surfaces with autologous cultured epithelium”, Lancet, 349, 990–993 (1997). PMID: 9100626.
