Statins are widely used for good reason. They reduce serum lipoprotein levels and treat dyslipidemias like atherosclerosis, and happen to have anti-inflammatory, anti-oxidative, anti-fibroproliferative, microvasculo-protective and neuroprotective effects too. Almost a third of the US adult population are prescribed them, and cardiologists half-joke that they should be offered as a condiment at fast-food restaurants, as they’ve shown great benefit in reducing cardiovascular mortality and morbidity. They have prevented (or delayed) millions of heart attacks since their introduction – and it looks like they have another trick up their sleeve: reducing the risk of revitrectomy in patients who have undergone vitrectomy for rhegmatogenous retinal detachment (RRD).
The antifibroproliferative effect of statins piqued the interest of a group of Helsinki-based doctors and researchers, who knew from the work of Jules Gonin back in 1934 about proliferative vitreoretinopathy (PVR) – the intraocular fibrosis formation that is considered to be the worst-case scenario after VR surgery and necessitates re-operation. An agent that can prevent PVR would be of great benefit, but clinical evaluations of steroids, daunorubicin, 5-fluorouracil and many anti-inflammatory and anti-VEGF agents have all failed to show a significant benefit. Could statins succeed where the others have failed? After all, rabbit studies of statins in a glaucoma filtration model suggested that they had a beneficial effect. Would this trend hold in humans undergoing VR surgery? After all, many patients undergoing VR surgery will also be receiving statin therapy.
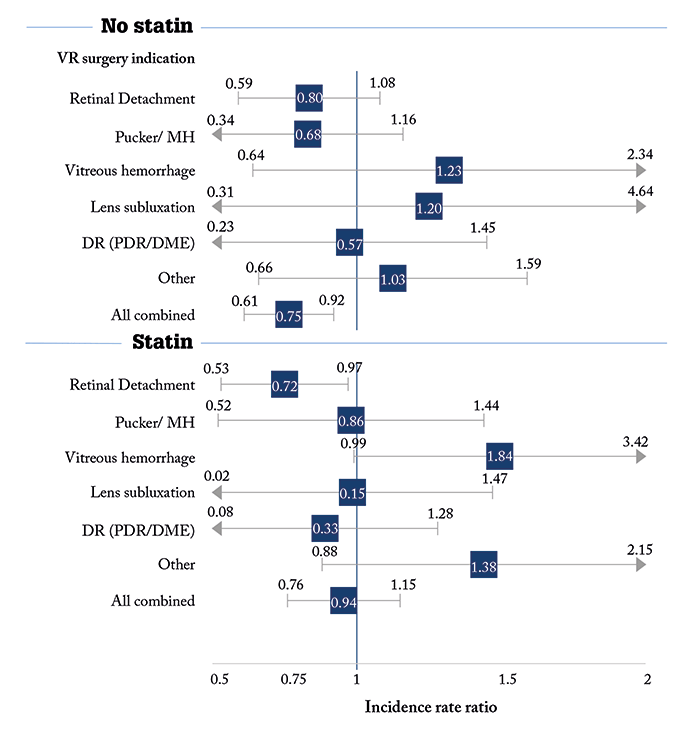
The team performed an exhaustive record review; after certain exclusion criteria were applied, the records of 5,707 patients aged ≥18 years who underwent vitrectomy in Helsinki University Hospital in Finland over a 6.5 year period between 2008–2014 were analyzed, including demographic variables, the type and duration of surgery, concomitant diseases, prescribed medications, and follow-up time. The primary end-point was revitrectomy during the 1-year postoperative follow-up period, due to retinal redetachment, vitreous rehemorrhage, postoperative endophthalmitis, recurrent pucker or unclosed macular hole. They found that RRD was the second most frequent indication for VR surgery (1,916 patients; 305 reoperations) – a rate of 0.20 (95% CI 0.18–0.23) per person-year. Patients who were on statin therapy at the time of operation had a lower relative risk of re-operation (an incidence rate ratio of 0.72, 95% CI 0.53–0.97; Figure 1), but not a lower absolute risk (incidence rate difference -0.58, 95% CI -4.30 to 3.15 for 100 person-years). They found no association with statin therapy and vitrectomy in the other VR subgroups (Figure 1). When they looked further at the statins used, of the three used in their cohort (simvastatin, atorvastatin, and rosuvastatin), only simvastatin seems to be associated with the lower revitrectomy rate. The study’s authors recognize that the comparison had a number of weaknesses; it was a registry-based trial, the operations were carried out by multiple VR surgeons and there was a possibility of confounding factors connected to statin use. But they do urge that further investigation be performed – to answer questions like: what age groups should receive the statins, how could statin therapy be best used as an adjuvant to prevent re-operations after RRD surgery, and should all patients diagnosed with an RRD start statin therapy right away? Frankly, it’s hard to ignore a 28 percent reduction in reoperation rates that can be achieved with a generic statin.
References
- S Loukovaara et al., “Statin use and vitreoretinal surgery: Findings from a Finnish population-based cohort study”, Acta Ophthalmol, [Epub ahead of print] (2018). PMID: 29338115.
