- Glaucoma specialists have been confused by POAG for too long, and this may be because it is actually multiple heterogeneous diseases
- It’s time we redefined the disease, and discuss the potential different subtypes and how best to manage them
- Patients with paracentral open-angle glaucoma should have low target IOPs, and patients with African-derived open-angle glaucoma should be screened and monitored alongside their children and family
- There remains much to learn, and we need to continue to find the different disease subtypes to better serve our patients
How long have we been confused about primary open-angle glaucoma (POAG)? The answer is a really long time, and even the term glaucoma is a misnomer; its origin stems from the ancient Greek, glaukos, which likely referred to the dull sheen or “glaze” of blindness that arises from a swollen cornea or cataract – both of which can be caused by chronic intraocular pressure (IOP) elevation (1). Fast-forward to the present day and there’s a myriad of terminology related to POAG that doesn’t reflect the complicated etiology of the disease (see sidebar: “POAG Terminology through Time”). Even the name “POAG” is bad because it fosters the suggestion that there is no secondary cause of the disease. But it needs to be secondary to something.
Pre-1970 terminology
- Chronic simple glaucoma
- Wide-angle glaucoma
- Glaucoma simplex
- Normal tension glaucoma
- Low pressure glaucoma
- High tension glaucoma
- Open-angle glaucoma
I think it’s time to take a new approach and redefine POAG. My goal is to break it down into its different components and really understand what is going on. Over many years of study, I have learned that it is likely to be several different diseases, and I believe the best approach is to identify the different subtypes and their unique risk factors. We have much to learn, but here is what we know so far...
Replacing “P” with “PC”
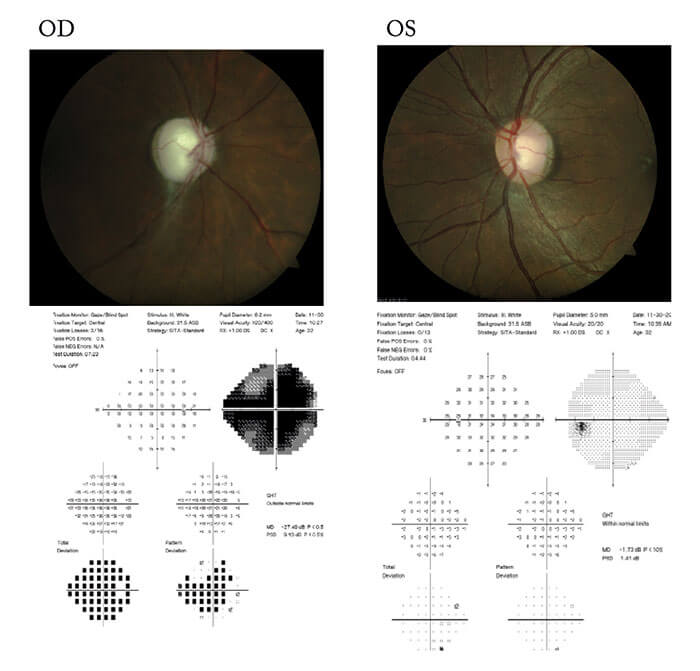
The first POAG subtype to treat as a separate entity is paracentral open-angle glaucoma (PC-OAG). The main feature of this subtype is that disease attacks the center of vision, causing early paracentral visual field loss. When studied by OCT, many of these patients have triangular defects in their pre-laminar optic nerve tissue – almost like a little man with a shovel dug out a trench in the superficial optic nerve head. Another feature of this subtype is that patients typically have lower IOPs than those observed in patients with peripheral visual field loss. However, because many patients with PC-OAG may also have pressures in the normal-tension glaucoma or high-tension glaucoma ranges (2), stratifying patients by IOP may not be serving us well. Instead, we should perhaps consider other biomarkers, such as disc hemorrhages, which have been found to present at a higher frequency in patients with PC-OAG (2). Genetically, we know that impaired nitric oxide (NO) signaling plays an important role in PC-OAG. Caveolin is a membrane protein that interacts directly with endothelial nitric oxide synthase (eNOS) to regulate NO production, and genomic variants in the CAV1/CAV2 region associate with the PC-OAG subtype (3). This, together with research showing that IOP is elevated and outflow facility reduced in eNOS knockout mice (4), means that we now know that NO signaling is very important for the regulation of IOP – and it’s why we’re seeing potential new drugs on the horizon that target NO and associated pathways. Until these drugs make it into the clinic, what are the options for patients with PC-OAG? A low target IOP is needed for these patients (16 mmHg is too high) and many may need even lower target IOPs in the range of 10–12 mmHg. Additionally, observational research suggests that dietary nitrates might favorably reduce the risk of POAG (5), particularly for PC-OAG, and there’s certainly no harm in trying to get patients to eat more leafy green vegetables!Replacing “P” with “AD”
The next POAG subtype is a very important entity and one we should be recognizing more: African-derived open-angle glaucoma (AD-OAG). We think of POAG as a disease of the elderly, and a common misconception is that it won’t be found in people below the age of 40 years. However, research performed in South Florida has shown that at least one African-derived population is likely developing glaucoma a decade or two earlier than their Caucasian counterparts (6).Take for example a case of an African-American man with a positive family history of glaucoma (see box “A Case of AD-OAG”). Of note, this patient was only 32 years old, his IOPs weren’t particularly high (18–24 mmHg), central corneal thickness (CCT) measures were low, and there was no evidence of a secondary glaucoma upon examination. So what do we do differently for this type of patient? We get them and their family on our radar – they’re all high-risk patients who need intervention, even their children. Research into the genetics of AD-OAG is spearheaded by Joan O’Brien, Mike Hauser and others. Constance Okeke is championing the effort to educate all patients about the importance of a positive family history of glaucoma, with emphasis on counseling young AD-OAG patients that their siblings and even their children should be under ophthalmic surveillance.
Background
- Male, 32 years old
- Last eye exam was eight years ago; routine eye exam revealed loss of vision in right eye
- Patient knew OD vision was “weak,” but it hadn’t been affecting his daily activities
- Vision: 20/600 sc OD; 20/20 sc OS
- IOP: 24 mmHg OD; 18 mmHg OS
- CCT: 512 µm OD; 522 µm OS
- Slit lamp examination unremarkable
- Gonioscopy: Gr III open 360° OU with 1+PTM
But we can do more – we need to deepen our understanding of AD-OAG because there are pathophysiological differences between African-Americans and Caucasians. For instance, a well-executed study examined over 10,000 disc photographs and showed that people of African descent had a reduced risk of disc hemorrhage compared with Caucasians (7). This is intriguing because disc hemorrhages are a structural biomarker for disease progression; yet, they occur less commonly in African Americans with open-angle glaucoma who are more likely to go blind from glaucoma than Caucasians with open-angle glaucoma. The reasons for this tendency are currently unclear, but these findings suggest that retinal hemodynamics might differ between these two populations, and we need to understand more so that we can improve the care of our patients.
Going backwards to move forwards
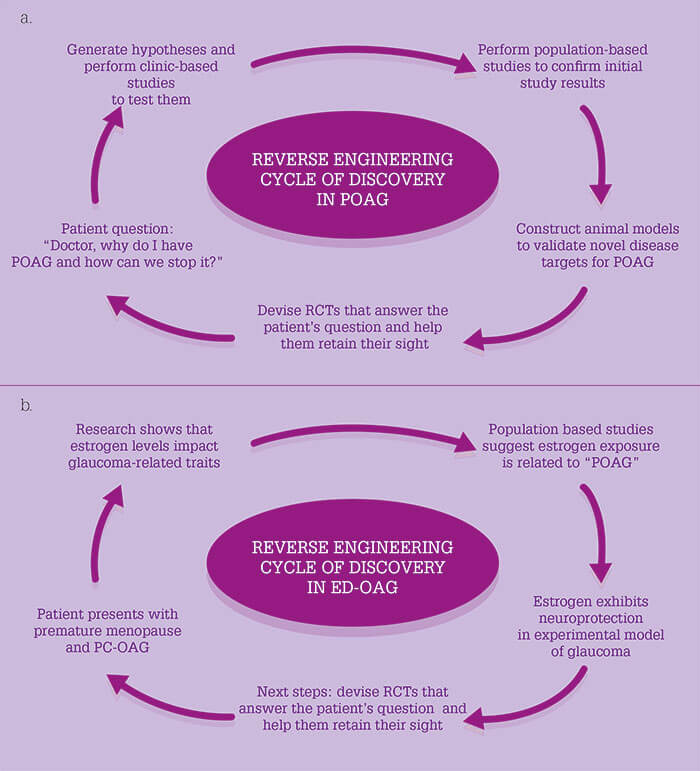
As PC-OAG and AD-OAG subtypes have unique themes, putting these patients together in one basket with other patients with glaucoma means we’d be missing opportunities to learn more about each of them. To find new drug targets for POAG, we need to entertain candidate mechanisms of the disease – of which there are many (see box: “Candidate Disease Mechanisms for POAG”). The best way to identify these might be through a reverse engineering cycle of discovery (Figure 1a). The following case helps demonstrate the concept of reverse engineering:- At 47 years of age, a female patient was identified as a glaucoma suspect because of cupping.
- Her OB/GYN history, which many of us ophthalmologists tend to ignore, was remarkable. She experienced pre-eclampsia at 30 years of age, and underwent a hysterectomy and bilateral oophorectomy at the age of 53.
- At 61 years of age, the patient noticed she’d lost the superior part of her visual field in her right eye. IOP was 21 mmHg in both eyes and examination showed excavation of the neuroretinal rims in both eyes, but worse in the right eye.
- Visual field assessments showed superior paracentral scotoma in the right eye, and superior nasal step and nasal step in the left eye.
You may be thinking “this sounds just like the PC-OAG subtype,” but I would like to draw attention to her GYN history and mention that estrogen is a big driver of NO synthase 3 activity. It also has a role in glaucoma-related traits, as indicated by several studies: retinal ganglion cells express estrogen receptors (8), and optic nerve structure and retinal sensitivity on visual field tests both vary as a function of the menstrual cycle (9, 10); IOP decreases during pregnancy despite the fact that CCT increases in the third trimester (11); and a post-hoc analysis of a randomized controlled trial (RCT) has shown that post-menopausal estrogen hormone therapy was associated with lower IOP (12). But what about POAG? Several studies indicate an increased risk of the disease in women with a reduced estrogen exposure during their lifetime (13)(14)(15)(16)(17)(18)(19). Estrogen has also been shown to preserve visual function and structure in a rat model of open-angle glaucoma (20). So when looking at the reverse cycle of engineering, this patient might actually have estrogen-deficient POAG (ED-OAG) because of her GYN history (Figure 1b), and the next steps would be to figure out how to leverage this information to design RCTs that answer the question of how we can help this patient safely improve the local estrogen levels in her eyes and retain her sight. Of course, we are far from being able to achieve that goal at the current time.
- Endothelial cell dysfunction with impaired NO signaling
- Estrogen deficiency
- Mitochondrial dysfunction
- Neuroinflammation
- Insulin resistance
- Oxidative stress
- Ocular connective tissue disorder
Some parting thoughts
Clinically, there is much to think about with POAG, and we need to see the bigger picture. POAG may be associated with increased IOP, but the majority of patients with the disease do not have IOPs exceeding 35 mmHg. So when confronted with a patient with “POAG” who has very high pressures, you should be considering other causes of elevated IOP such as steroid exposure or exfoliation. Physiological cupping should also be treated with caution because many genetic markers for a large cup-to-disc ratio are also markers for POAG (21), and these patients should be monitored for the development of glaucoma. In the future, we’ll hopefully know the full complement of genes dictating optic nerve cup and shape, and be able to use this information to predict which patients might get POAG. Caution should also be merited in cases of rapid visual field progression because this is rare in patients with POAG – if you’re seeing this, seek alternative causes for rapid progression. Asking about steroid use, and eye rubbing could be informative. Performing a diurnal curve to look for IOP spikes and neuroimaging might also reveal why some patients are deteriorating at a rapid rate.Let’s help our patients…
I’ve defined subtypes of POAG that I believe exist. They each have different features, different genetic markers that point to specific biochemical pathways in their disease – different etiologies that dictate how best they should be managed. To achieve a precision medicine approach to treatment, we need to perform more research to find all the different subtypes, and we need to accept that some patients may have overlapping features of several different subtypes of the disease. Let’s help our patients by taking the “P” out of POAG. I said that it has to be secondary to something – in fact, it is secondary to many things.Louis Pasquale is Director of the Glaucoma Service and Telemedicine at Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, USA. Financial disclosures: Paid Consultant for Bausch + Lomb and Eyenovia. Travel support from The Glaucoma Foundations in New York and San Francisco.
References
- N Mantzioros., Available at: http://bit.ly/HistoryGlaucoma. Accessed February 7, 2017. SC Park et al., Ophthalmol, 118, 1782–1789 (2011). PMID: 21665283. SJ Loomis et al., Ophthalmol, 121, 508–516 (2014). PMID: 24572674. Y Lei et al., Invest Ophthalmol Vis Sci, 56, 4891–4898 (2015). PMID: 26225628. JH Kang et al., JAMA Ophthalmol, 134, 294–303 (2016). PMID: 26767881. CL Bokman et al., PLoS One, 30, e115942 (2014). PMID: 25549331. A Skaat et al., Ophthalmol, 123, 1476–1483 (2016). PMID: 27117781. C Munaut et al., Br J Ophthalmol, 85, 877–882 (2001). PMID: 11423466. ME Akar et al., Acta Ophthalmol Scand, 82, 741–745 (2004). PMID: 15606474. I Yucel et al., Can J Ophthalmol, 40, 51–57 (2005). PMID: 15825530. K Green et al., Ophthalmic Res, 20, 353–357 (1988). PMID: 3237393. TS Vajaranant et al., Am J Ophthalmology, 165, 115–124 (2016). PMID: 26940165. YE Wang et al., Ophthalmology, 123, 729–736 (2016). PMID: 26948305. LR Pasquale and JH Kang. Eye, 25, 633–641 (2011). PMID: 21336255. CA Hulsman et al., Am J Epidemiol, 15, 138–144 (2001). PMID: 11447046. TS Vajaranant et al., Menopause, 21, 391–398 (2014). PMID: 24061049. LR Pasquale et al., J Glaucoma, 16, 598–605 (2007). PMID: 18091177. PA Newman-Casey et al., JAMA Ophthalmol, 132, 298–303 (2014). PMID: 24481323. KS Na et al., PLoS One, 11, e106473 (2014). PMID: 25210892. K Prokai-Tatrai et al., Molecular Pharmaceutics, 5, 3253–3261 (2013). PMID: 23841874. BJ Fan et al., Invest Ophthalmol Vis Sci, 28, 1788–1792 (2011). PMID: 21398277.
