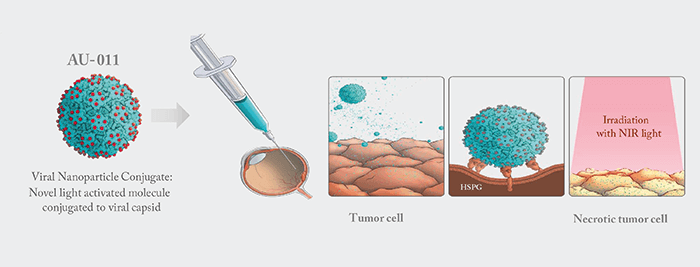
Choroidal melanoma represents a serious unmet medical need; despite receiving radiotherapy, 25 percent of patients with the disease develop metastatic disease after five years (1). Enter AU-011 – a viral nanoparticle conjugate comprising a novel light-activated molecule conjugated to a viral capsid that is currently under investigation. When injected into the vitreous, AU-011 binds to heparan sulfate proteoglycans on the surface of tumor cells. Stimulation with near-infrared light (689 nm) activates the drug, disrupting the tumor cell membrane and causing subsequent necrosis of the tumor (Figure 1).
Initial studies in rabbits have demonstrated that a therapeutic dose of 50 µg induced complete necrosis of melanoma in 80 percent of animals (2). But what about in humans? Carol Shields of Wills Eye Hospital, Philadelphia, USA, recently shared interim results from a Phase Ib/2 trial of AU-011 at the recent Annual Meeting of the American Academy of Ophthalmology in New Orleans (2). Six patients with small-medium choroidal melanoma (2–3.4 mm in thickness with evidence of documented growth or sub-retinal fluid) were enrolled into the open-label ascending single and repeat dose clinical trial, with three patients each receiving a single dose of 20 or 40 µg. The primary endpoint, safety by multimodal imaging, was met. Though no serious adverse events were found, Shields reported that there was some inflammation in the anterior and posterior segment that led to increased IOP in three patients. “We wonder if this could be a sign of new stimulation from this medication,” said Shields. Visual acuity for all patients was preserved within five letters of their pre-treatment vision.
The secondary endpoint was preliminary efficacy at 3–6 months, assessed by B-scan ultrasonography of tumor height. Five of the six patients showed stable disease and one patient showed tumor growth that required plaque radiotherapy. Shields also noted other related findings to the treatment: “tumor change in color, loss of orange pigment, loss of melanin and reduction in macular fluid.” Shields closed her presentation with a nod to the scientists behind AU-011. “I’d like to acknowledge the scientist John Schiller, inventor of the HPV vaccine, who modified that technology to adapt it to this new drug, as well as Elisabet de los Pinos – the mastermind behind this drug, who is taking it from bench to bedside.”
References
- M Diener-West et al., “Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26.”, Arch Ophthalmol, 123, 1639–1643 (2005). PMID: 16344433. CA Shields, “A Phase 1b clinical safety study of a novel tumor targeted therapy (AU-011) for the treatment of primary choroidal melanoma”. Presentation at the American Academy of Ophthalmology; November 11, 2017; New Orleans, USA.
