The high incidence of dry eye disease (DED) is well known, and its impact on quality of life is increasingly appreciated. Patients must not only endure discomfort but also accept impairments in performance of normal tasks: for example, reading speed is reduced by 14 percent in DED patients (1), and dry eye symptoms are exacerbated by reading (2). To add insult to injury, many patients cannot tolerate first-line DED therapies – cyclosporin emulsions – because of formulation derived side effects, particularly burning sensation after installation. Furthermore, these formulations are associated with large drop volumes (causing spill-over from the eye) and poor corneal spreading and retention time (reducing uptake efficiency). Hence, they do not provide optimal bioavailability and onset speed – so even when patients accept cyclosporin, they do not see its full benefit.
Novaliq’s new drug in development, CyclASol – 0.1 percent cyclosporin A in a semi-fluorinated alkane (SFA) vehicle – is designed to unleash the full potential of cyclosporin A in DED treatment. The SFA formulation enhances corneal residence time and corneal coverage, providing better bioavailability and faster onset. Furthermore, SFA physicochemical characteristics result in smaller drop sizes, which avoids drug loss through overflow from the eye (3). Critically, SFAs do not allow the side effects associated with oil-based vehicle, e.g. blurred vision. Finally, SFAs are water free and therefore obviate preservatives.
These benefits are not simply theoretical: a recent proof-of-concept study reported strong CyclASol-mediated benefits in DED patients (4). But can this be replicated in larger cohorts?
ESSENCE
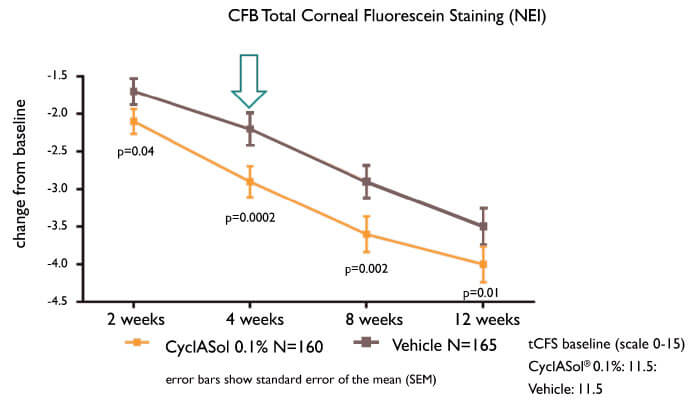
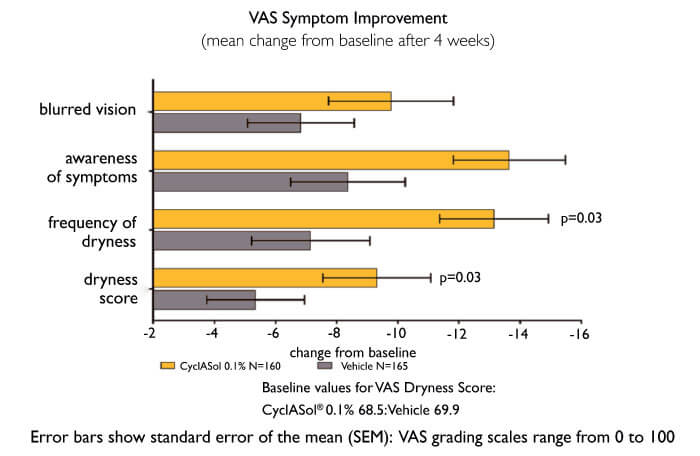
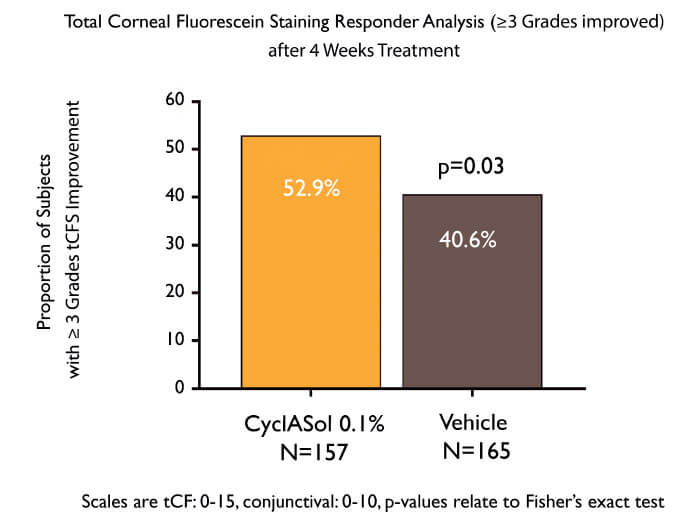
To answer this question, the ESSENCE clinical study compared twice-daily CyclASol with SFA alone in 328 patients with aqueous-deficient DED. The results were unequivocal (5): CyclASol achieved the primary endpoint (four-week efficacy as measured by corneal staining) and was clearly superior to vehicle alone (p=0.0002). The effect on staining was most marked in the central cornea, where disturbances can affect vision. Furthermore, CyclASol showed early onset of action (two weeks), and maintained its benefit over the entire study (three months), while demonstrating an exceptional tolerability profile (instillation site reactions: ~2.5 percent). Moreover, statistically significant improvements in a prespecified symptom endpoint (Dryness Score) at Day 29 were demonstrated in the CyclASol 0.1 percent treatment group compared to the vehicle group.
ESSENCE data are reinforced by results from a Phase II trial of CyclASol in moderate-to-severe DED patients (6), which compared CyclASol with (i) vehicle and (ii) the approved cyclosporin emulsion Restasis (Allergan, Irvine, CA). In brief, CyclASol-treated patients had lower corneal and conjunctival staining than either comparator. Furthermore, like ESSENCE, this trial showed that CyclASol mediates a two-week onset of action and improves in particular the central corneal area – all with low adverse event rates. The investigators concluded that CyclASol is safe, tolerable and has a faster therapeutic effect than Restasis.
Thus, CyclASol data indicate a brighter future for DED patients. But what do physicians think? We asked John Sheppard.
John Sheppard, President, Virginia Eye Consultants, works at a large practice comprising 200 employees and 20 doctors spread over f ive locations. Sheppard frequently treats patients with DED.
Today, a mainstay of DED management is topical cyclosporin, which acts to modulate the T cell-based inflammatory processes common in dry eye. Prior to the approval of Restasis, many cornea specialists resorted to compounded 1 percent to as high as 4 percent preparations of cyclosporin A in disgustingly viscous preparations of peanut oil, canola oil or medium chain triglycerides. The drops were gigantic and the containers always sticky. Even currently approved emulsion oil-based formulations have drawbacks: with 40-50 microliter drop volumes, a proportion of the dose spills out of the eye (the ocular surface only holds about 20 microlitres). Also, their limited ability to spread over or remain adherent to the corneal surface results in relatively low drug availability. Thus, formulation shortcomings mean that the potential benefit of cyclosporin A is never fully realized.
That’s why ESSENCE was so welcome – it tested a unique, SFA-based cyclosporin formulation. Being water-free, SFA efficiently solubilizes the hydrophobic cyclosporin molecule and is immune to microbial growth (hence, requires no preservatives). In addition, it has a low surface tension, which assists corneal coating and retention (residence time: up to 240 minutes), suggesting enhanced drug bioavailability and efficacy. Finally, SFA drops are smaller (~10 microlitres) than those of water or oils, thereby avoiding overflow and waste.
ESSENCE demonstrated that twice-daily treatment with SFA cyclosporin formulation mediated a remarkably early benefit (two weeks) in moderate-to-severe DED patients. This was sustained throughout the trial (12 weeks) resulting in faster reading speeds in DED patients. Notably, CyclASol’s side-effect profile was far better than those of standard DED products. Remember, nearly a quarter of patients on Restasis and similar products end up refusing the drugs. Such sub-optimal tolerability results in additional clinic visits, unnecessary expense in the healthcare system, and patient discontent. The great promise of CyclASol is that providers can prescribe it to patients and remain confident that these side-effects – and consequent therapy non-adherence – are unlikely. And that profile could dramatically change our approach to first-line DED medication.
For more information regarding CyclASol, visit www.novaliq.com

References
- P Mathews et al., “Functional impairment of reading in patients with dry eye”, Br J Ophthalmology, 101, 481 (2017). PMID: 27450145. S Karakus et al., “Effects of prolonged reading on dry eye”. Ophthalmology, 125, 1500 (2018). PMID: 29705055. P Agarwal et al., “Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics”, Int J Pharm, 538, 119 (2018). PMID: 30831252. GL Torkildsen, “A clinical phase 2 Study to assess efficacy, safety, and tolerability of CyclASol for treatment of Dry Eye Disease”. Poster presented at the AAO Meeting; October 2017; New Orleans, 2017. Poster #59. Novaliq press release October 18, 2018: “Novaliq announces positive topline results for its Cyclasol Phase 2b/3 Essence trial in patients with dry eye disease”. D Wirta et al., “A clinical phase II study to assess efficacy, safety, and tolerability of CyclASol for treatment of dry eye disease”, Ophthalmology, in press, doi: 10.1016/j.ophtha.2019.01.024 (2019). PMID: 30703441.
