Geuder has championed innovation ever since its inception. It pioneered the first sterile atraumatic suture needle for corneal surgery, the first motorized keratoplasty trephine, the first dual blade vitrectomy cutter… And now, the first transportation and injection system for Descemet lamellas in a ready-to-use cartridge: the DMEK RAPID (Revolutionary Advanced Preloadable Injection Device). Designed in cooperation with Professor Peter Szurman (Knappschaftsklinikum Saar Sulzbach, Germany) and the German Society for Tissue Transplantation (DGFG), the DMEK RAPID boasts a unique preloaded design, allowing human tissue to be delivered to the OR in a ready-to-use condition for the first time in transplantation history.
As with all Geuder products, its genius lies in its simplicity. In this case, the elegant, preloaded system that reduces OR time by outsourcing the risky and time consuming preparation of Descemet lamellas to the eye bank. By allowing physicians to focus on implantation only, the device eliminates the long learning curve associated with conventional surgery methods and facilitates DMEK’s continued standardization.
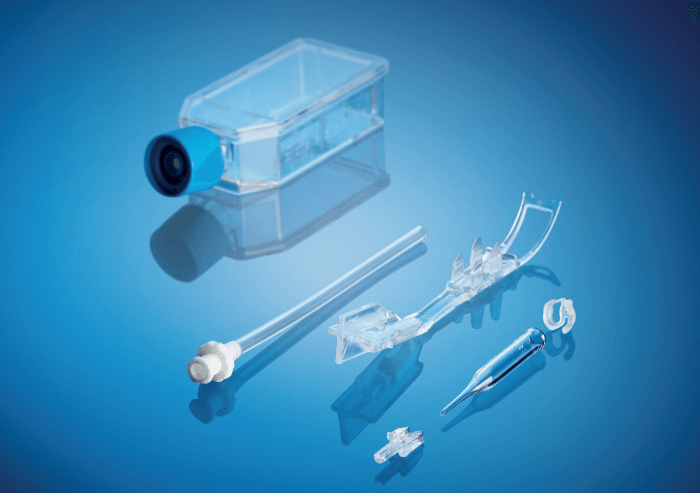
The set consists of a glass cartridge for loading, carrying and injecting the prepared Descemet lamella; two permeable plugs to hold the lamella in place within the cartridge, while enabling constant exchange of nutrient solution; a transportation holder that keeps the cartridge in place within the flask; and a connection tube for touch-free loading of the Descemet lamella into the cartridge.
The system will be available in two designs, for both cold and warm storage. Though approval for cold storage is still pending, the DMEK RAPID transportation set for warm storage has been CE-certified since June 2019. Countless physicians and patients have already benefited from faster, safer and more predictable DMEK surgeries – and it is time for more to do the same.
