Drugs almost always fare better in clinical trials than they do in the real world. Real-world patients do not comply as closely with treatment regimens; they often have comorbidities that would see them excluded from a trial; and they have a tendency to miss appointments. In the UK, the real world has an additional confounding factor: a policy from the National Institute of Health and Clinical Excellence (NICE), which is the public body that advises on how drugs should be used.
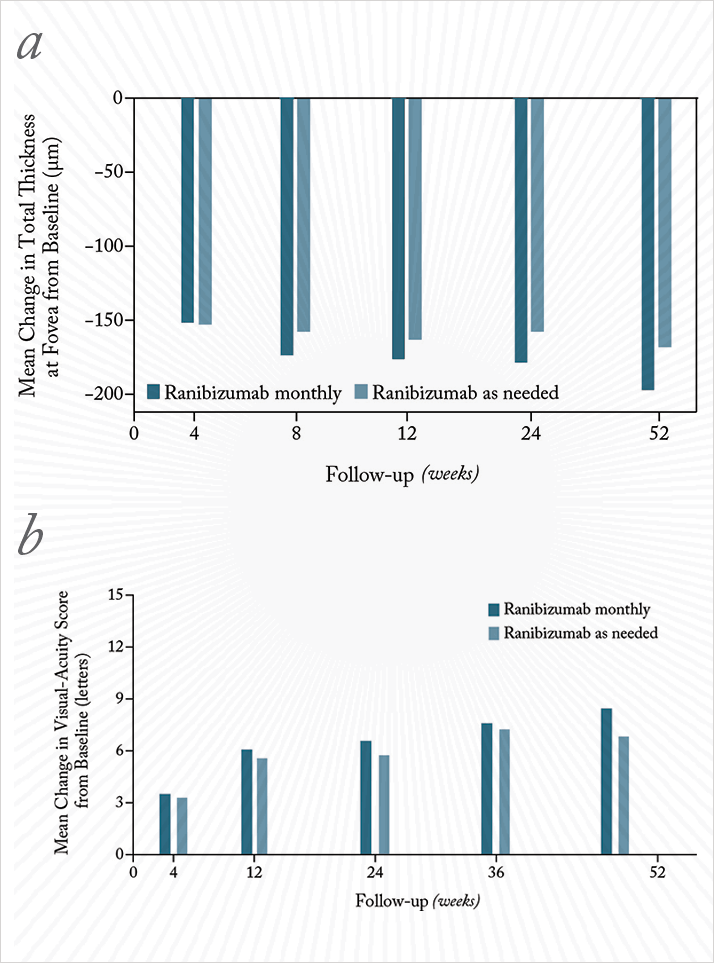
In the name of cost-saving, NICE advises that for the treatment of neovascular age-related macular degeneration (nAMD), ranibizumab should be administered far less frequently than the pivotal studies that got the drug approved in the first place (2,3). The trials used monthly ranibizumab injections for two years; NICE advise that ranibizumab therapy be administered as a loading phase of three injections given at monthly intervals, followed by pro re nata (PRN) treatment if active disease is detected at monthly assessment visits. This so-called inject-and-extend protocol is based partly on the fact that trials of monthly versus PRN dosing have shown that (under strict trial conditions) inject-and-extend therapy is only slightly (but statistically significantly) worse than continuous monthly ranibizumab dosing (Figure 1; 4).
The UK also has an excellent central electronic medical records (EMR) system for ophthalmology. This enabled researchers from UK Age-Related Macular Degeneration EMR Users Group to perform a multicenter, national nAMD database study on the records of 11,135 patients that comprised 92,976 treatment episodes on 12,951 eyes, over a period of five years (5) to determine if the performance of inject-and-extend seen in the trials exists in the real world. In comparison with the pivotal trials which used a monthly dosing regimen (2,3), the ‘inject-and-extend with PRN’ approach resulted in lower visual acuity (VA) gains, with vision tailing off after the peak gain, and a lesser proportion of patients who gained at least 15 letters of vision or maintained vision of 20/40 or better.
The upside of the findings is that the vision gains that were achieved according to NICE’s recommended regimen were accomplished with fewer injections and hospital visits than in the pivotal studies. Looking at the data, the authors suggested that “the proportion of patients with VA of 20/40 or better will reflect not just the efficacy of the treatment but also how quickly patients can access the treatment,” and warned of the problems that “capacity constraints that prevent intended monthly review at some centers” can have on patients’ vision. One take-home message from this analysis seems to be: if you have a patient on PRN ranibizumab dosing, make sure that patient is seen monthly.
References
- National Institute of Health and Clinical Excellence TA155 Macular degeneration (age-related) - Ranibizumab and Pegaptanib: Guidance. http://www.nice.org.uk/nicemedia/live/12057/41719/41719.pdf. Accessed February 3rd, 2014. P. Rosenfeld et al., “MARINA Study Group. Ranibizumab For Neovascular Age-Related Macular Degeneration”, N Engl J Med., 355, 1419–31 (2006). D. Brown et al, “ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration”, N Engl J Med., 355, 1432–44 (2006). CATT Research Group, “Ranibizumab and bevacizumab for neovascular age-related macular degeneration”, N Engl J Med., 364, 1897–908 (2011). Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group, “The Neovascular Age-Related Macular Degeneration Database: Multicenter Study of 92 976 Ranibizumab Injections: Report 1: Visual Acuity”, Ophthalmology, Epub ahead of print (2014). doi:10.1016/j.ophtha.2013.11.031.
