With Frank Chen, Associate Director, Santen Ventures, US
Gus Gazzard, Consultant Ophthalmic Surgeon and Glaucoma Service Director at Moorfields Eye Hospital, London, UK
Mark Packer, President of Packer Research Associates Inc., Boulder, Colorado, US
Advanced technologies do not simply appear, fully formed. They are almost always the result of multiple innovations, careful iterative design, and the hard work of multidisciplinary teams and collaborations across business and academia. The most effective collaborations recognize that beyond the initial allure of the technology itself, numerous factors influence the rate of this acceleration and ultimately – the chance of success. The scientific evidence, market need and indeed the teams and diverse mindsets that constitute a joint venture all play a part.
Combining forces in this way propels individual technologies forward – and provides new solutions to address today’s patient needs. But what about the big picture? And how do we drive the whole eye care industry forward in the long term?
Frank Chen, Associate Director at Santen’s investment arm, Santen Ventures, US, is well aware of the journey from emerging innovation to validated technologies. “We take on the role of strategic investor – investing in companies that traditional venture capitalists may not find attractive; for example, companies that are creating interesting solutions but don’t necessarily have a concrete business model.” Such a strategy, explains Chen, allows us to aim for “the ideal ophthalmic future,” rather than having to focus on short-term return on investment. “Start-ups, for example, have the advantage of being singularly focused on one product and therefore can move extremely fast,” says Chen. “In turn, we can apply different use cases in different regions, which can help drive the success and expansion of start-up technologies.”
So how does this play out in real time and what advanced technologies are helping to shape the present and future of ophthalmology? Over the following paragraphs, we explore two examples, where strong collaborative approaches are championing innovation that can make a real difference for patients now – and in the future.
The first, the potential for direct selective laser trabeculoplasty (DSLT) to be used to provide appropriate solutions to address the long-standing issue of glaucoma patient adherence to eye drop regimens. The second focuses on intraocular lens (IOL) design and how advances in this space are opening the door to good intermediate visual acuity without glasses to 90% of cataract surgery patients not currently using multifocal IOLs (1, 2).
Next Generation Glaucoma Intervention
Patient adherence to eye drop regimens has been a long-standing issue in glaucoma treatment – and attention is increasingly turning to advanced technologies to address this. The pioneering LiGHT study has already established selective laser trabeculoplasty (SLT) as a first-line treatment option for glaucoma (3). Here, Gus Gazzard, Consultant Ophthalmic Surgeon and Glaucoma Service Director at Moorfields Eye Hospital, London, UK, considers if DSLT – an automated, extremely quick procedure to lower IOP – represents the next step forward in glaucoma intervention.
“The biggest barrier to standard glaucoma treatment – medication in the form of drops – is patient compliance,” says Gazzard. Over 50 percent of patients don’t take medication as they are advised (4, 5).
“The treatment burden for patients can be huge – they may experience local and systemic side effects, and they have to remember to take the medication every day – a constant reminder that they suffer from glaucoma,” Gazzard explains. Local and systemic toxicity of the medications is also a significant issue, and can result in not only ocular pain and irritation, but also reduced exercise tolerance or respiratory symptoms (6). So what can be done for glaucoma patients to reduce the impact of medication?
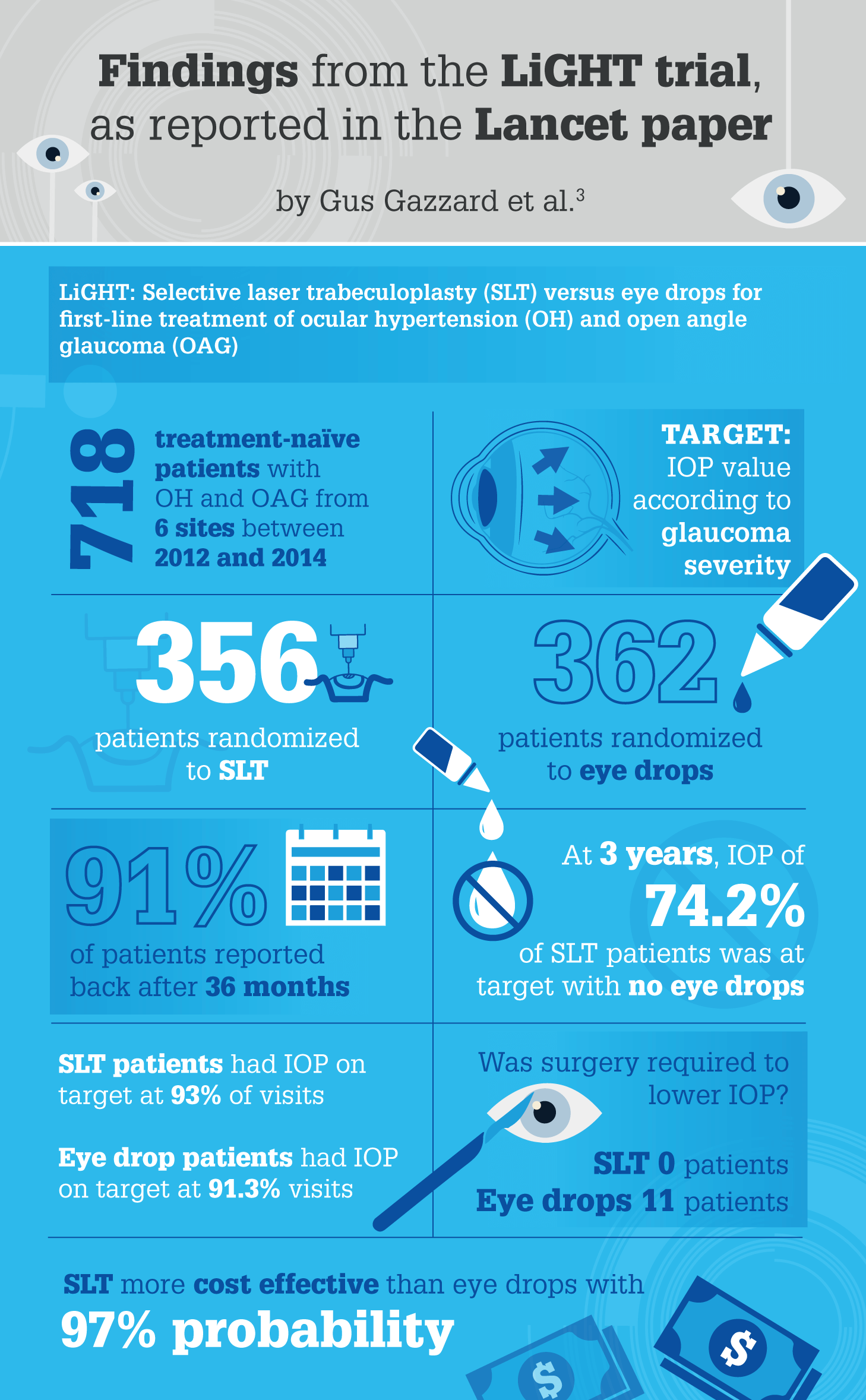
The LiGHT study, conducted at Moorfields Eye Hospital, is an ongoing project that reports the outcomes of 718 glaucoma patients (3). Funded by the National Institute of Health Research (NIHR) in the UK, LiGHT compared treatment pathways, beginning with either SLT or eye drops to lower the IOP. “Health-related quality of life was the primary outcome measure,” says Gazzard. “And we also looked at the intensity of the treatment, whether the laser treatment could be repeated, how effective it was, and whether it preserved vision as well or better than eye drops.”
Of the four main papers produced as a result of the study so far, the most significant – published in March 2019 in The Lancet (3) – showed that patients on the pathway beginning with SLT were controlled at a predefined target pressure. “At 36 months, approximately 75 percent of patients had IOP controlled using laser treatment alone,” reports Gazzard. “In short, SLT worked very well for those patients.”
According to Gazzard, the remaining three papers showed that:
- SLT was effective at lowering pressure, and there was a 28 percent reduction in IOP two months after initial laser in treatment-naïve patients, just as there was with prostaglandin eye drops (7).
- The laser treatment could be successfully repeated when the original laser effect wore off. In fact, the second treatment was often more successful than the first, suggesting that some patients might need a top-up treatment for adequate pressure control (8).
- Patients’ visual fields showed less damage in the laser-treated group than in the drop-treated group, so it seems that the group treated with SLT first had better visual field preservation. This is the first time that such an effect has been shown, and may be due to patient compliance issues with drops (9).
“Now, DSLT – an innovative treatment being developed from a collaboration between Santen and BELKIN Laser – has opened the possibility of improving glaucoma patient outcomes even further, by allowing SLT to be delivered regularly to patients by technicians, rather than surgeons,” says Gazzard. “This quick, gentle treatment could be administered regularly, with the potential to further improve glaucoma patients’ outcomes.”
DSLT externally fires a laser straight through the outer layers of the eye into the tissue concerned. It automatically identifies the position of the external limbus using advanced gaze tracking software and delivers 100 to 120 laser pulses in one second. “The delivery is much quicker and therefore more acceptable to the patient,” says Gazzard. “And it is essentially pain free.
“Further investigation is needed, but it is possible that DSLT could remove one of the biggest costs in the equation of regular glaucoma treatment: the surgeon’s time. With the proof of concept established, and trials ongoing, the future could see a quick, straightforward, regularly reapplied treatment for IOP control.”
The World at Arm’s Length
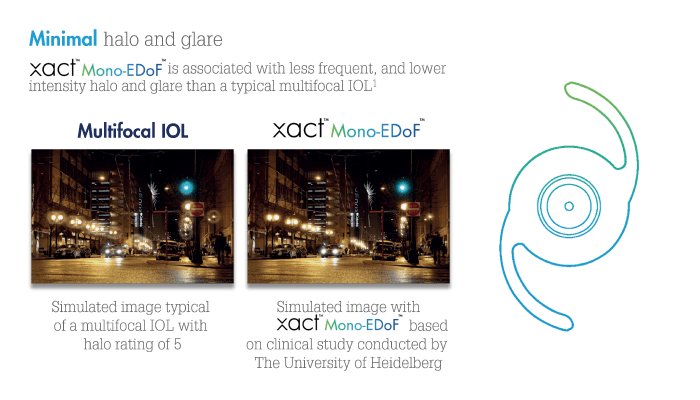
When it comes to advanced technology that is already having a positive patient impact, the xact™ Mono-EDoF™ IOL, developed by Santen in collaboration with Advanced Vision Science, is a clear example. With its distinctive optical design, the new monofocal IOL – with Extended Depth of Focus (EDoF) feature – offers continuous focus from distance to intermediate, with minimal glare and halos compared with multifocal solutions (1, 2, 10, 11).
“The idea of a Mono-EDoF™ lens hits a much-needed compromise between quality of vision and seeing well without glasses in any circumstances,” says Mark Packer, President of Packer Research Associates Inc., Boulder, Colorado, US. “Up until now, patients who wish to be spectacle-independent have had to use multifocal, diffractive lenses, which produce unwanted side effects, such as halo or glare, and decreased contrast sensitivity.” The resulting lens has contrast sensitivity similar to that of traditional monofocal IOLs, with no increased incidence of unwanted optical side effects. “These aspects were studied in a number of different scenarios using software simulations,” Packer says. “And based on these findings – and the fact that this IOL meets the optical standard for a monofocal lens – it was given a CE mark.”
Despite its apparent simplicity, the xact™ Mono-EDoF™ IOL is the result of a clearly defined concept and careful design. Exhaustive testing of the xact™ Mono-EDoF™ IOL’s intermediate range was necessary. “We didn’t just measure one distance, but checked the results at 50, 60 and 70 cm,” says Packer. The team found that patients’ visual acuity at arm’s length ranged from 20/25 to 20/30, which meant that most daily activities, such as using electronics, cooking – even reading a list of ingredients on a packet – could be done with no glasses. “And at the 20/30 range, patients might even be able to read a book with no spectacles in the right lighting conditions.” That said, notes Packer, the team was not striving for perfect near vision – and patients should be advised that they are likely to need reading glasses to read a book or a newspaper (1, 2, 10).
“Good-quality vision at the intermediate range, commonly used in today’s world – the world at an arm’s length, as I call it – is the range patients want and need to access without glasses, and this is what the xact™ Mono-EDoF™ IOL provides,” explains Packer. “People who are motivated to get rid of glasses when performing activities such as driving, using screens, doing crafts (pottery, needlepoint, scrapbooking) are great candidates for this lens.”
For a person who never wants to wear glasses – even to read small print on a medicine bottle? “A trifocal lens might be a better option. But those patients have to be prepared for a trade-off: possible dysphotopsias, halo and glare – and, of course, a higher price.”
The xact™ Mono-EDoF™ IOL may be new – but it benefits from decades of expertise; the material used was actually developed by Santen over 20 years ago with the specific aim of eliminating glistenings, which can reduce patients’ quality of vision. Over the past 10 to 15 years, there has been a huge change in the design of multifocal lenses, from bifocal to trifocal lenses to EDoF lenses. However, dysphotopsias and halos have been a real problem for patients, which is why the current multifocal lens market adds up to only around 10 percent of all cataract surgeries. And that’s why a new type of IOL has been developed; monofocal EDoF lenses promise to give patients increased depth of focus with reduced risk of dysphotopsias and halos.
The polymer material used for xact™ Mono-EDoF™ IOL was tested in practice for many years. It is hydrophobic, which means that it has a higher refractive index, and therefore a thinner lens. It is resistant to scratching on insertion, and, unlike some other hydrophobic lenses, it does not develop intralenticular glistenings (1, 11).
The number of diffractive rings on an IOL is important – the more rings there are on the lens surface, the more likely it is for patients to experience glare and halo. With only four rings, the xact™ Mono-EDoF™ lens has a depth of focus of around 1.5 D (1, 2, 10, 11, 12). It is also insensitive to tilt and decentration, so optical performance isn’t degraded.
It is clinical opinion that as the biometric characteristics of the lens are also a little different from typical monofocal lenses, the surgeon can aim for emmetropia rather than minimal myopia. At present a toric version is not yet on sale so patients should have less than 0.75 D of astigmatism to be suitable candidates. Interesting possibilities could arise to increase the depth of focus by using this IOL in the dominant eye with monovision, or mix and matching this lens with a trifocal lens in the other eye for patients who want more spectacle independence for near vision (1).
Packer concludes, “The lengthy and robust development process resulted in a lens that is crystal clear, with a smooth, mirror-like finish thanks to the material’s hardness (the material is the same as used in enVista® lens from Bausch+Lomb). Any traditional imperfections, such as scratches or fold marks, which were the result of the surgeons’ handling of the lens, do not occur with this IOL (13, 14).”
Future-Proofing Ophthalmology in a Changing World
The world is constantly changing and Frank Chen from Santen believes that the pace of change will only accelerate. “The current COVID-19 pandemic is a good reminder that we must always think ahead and plan for the future,” he says. “Home health monitoring and telemedicine, for example, could well become the new normal. There is good evidence that both solutions not only lower health care costs, but also empower patients.”
“Investment in the technologies emerging out of immediate need, will ultimately help connect patients to physicians in any circumstance,” says Chen. “And build better datasets for research, public health, and insight generation in the future.”
It is clearer now more than ever, that intelligent collaborations that analyze, test and develop novel materials and technologies are essential to delivering solutions for the challenges that patient face today, and tomorrow.
PP-No product-EMEA-0005
Date of preparation: July 2020
References
- Data on file, Combined Clinical Data Analysis of CP7801 and CP7882 [ESR 7959]. 2019
- Data on file, The University of Heidelberg [AVS CP7882]. 2019
- G Gazzard et al., “Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial”, Lancet, 393, 1505 (2019). PMID: 30862377.
- DS Friedman et al., “Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS)”, Invest Ophthalmol Vis Sci, 48, 5052 (2007). PMID: 17962457.
- BL Nordstrom et al., “Persistence and adherence with topical glaucoma therapy”, Am J Ophthalmol, 140, 598 (2005). PMID: 16226511.
- DC Broadway, H Cate, “Pharmacotherapy and adherence issues in treating elderly patients with glaucoma”, Drugs Aging, 32, 569 (2015). PMID: 26136215.
- A Garg et al., “Primary selective laser trabeculoplasty for open-angle glaucoma and ocular hypertension: clinical outcomes, predictors of success, and safety from the Laser in Glaucoma and Ocular Hypertension trial”, Ophthalmology, 126, 1238 (2019). PMID: 31028768.
- A Garg et al., “Efficacy of repeat selective laser trabeculoplasty in medication-naïve open-angle glaucoma and ocular hypertension during the LiGHT trial”, Ophthalmology, 127, 467 (2020). PMID: 32005561.
- G Gazzard et al., “Selective Laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT”, Health Technol Assess, 23, 1 (2019). PMID: 31264958.
- Data on File, Augenklinik Rheine [AVS CP7801]. 2019
- I Baur et al., “The first clinical experience with MonoEDoF”, ESCRS. 2019
- xact™ Mono-EDoF™ Model ME4 device description
- M Packer et al., “Safety and effectiveness of a glistening-free single-piece hydrophobic acrylic intraocular lens (enVista)”, Clin Ophthalmol, 7, 1905 (2013). PMID: 24109169.
- P Heiner et al., “Safety and effectiveness of a single-piece hydrophobic acrylic intraocular lens (enVista®) – results of a European and Asian-Pacific study”, Clin Ophthalmol, 8, 629 (2014). PMID: 24729678.
