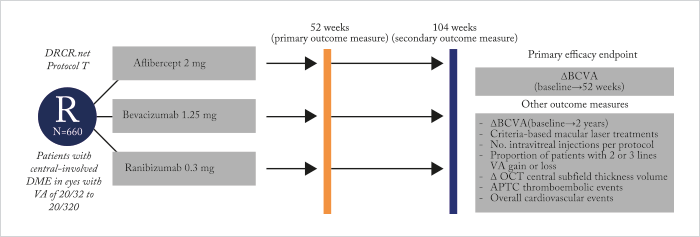
Last year saw the publication of the one-year Protocol T trial results (1). Six hundred and sixty patients with diabetic macular edema (DME) were randomized to receive intravitreal aflibercept, bevacizumab or ranibizumab (see Figure 1). All drugs performed well, but aflibercept did best of all – its use was associated with significantly greater improvement in best-corrected visual acuity letter scores from baseline levels than either of the other two drugs – but that didn’t tell the full story. For eyes with relatively good vision at baseline (78 to 69 letters; Snellen equivalent, 20/32–20/40), aflibercept-receiving patients did as well as patients receiving either ranibizumab or bevacizumab. But in patients with worse baseline vision (<69 letters; Snellen equivalent, 20/50 or worse), aflibercept use gave patients significantly greater VA improvements (from baseline levels) than patients who received bevacizumab or ranibizumab (see Figure 2a).
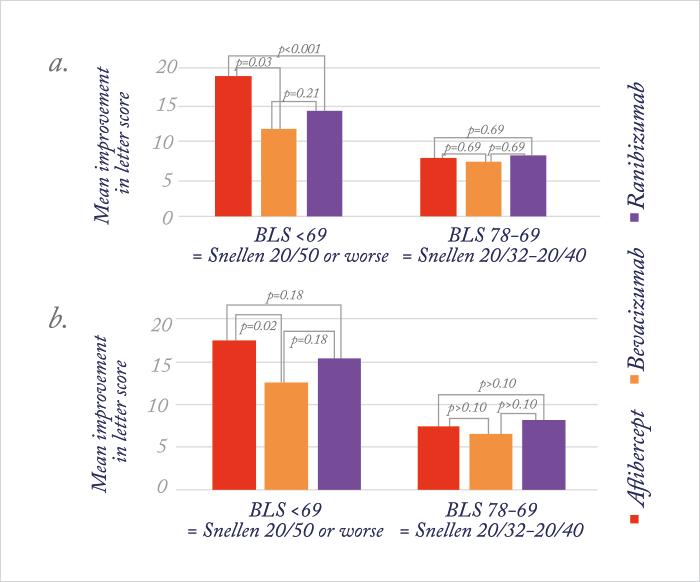
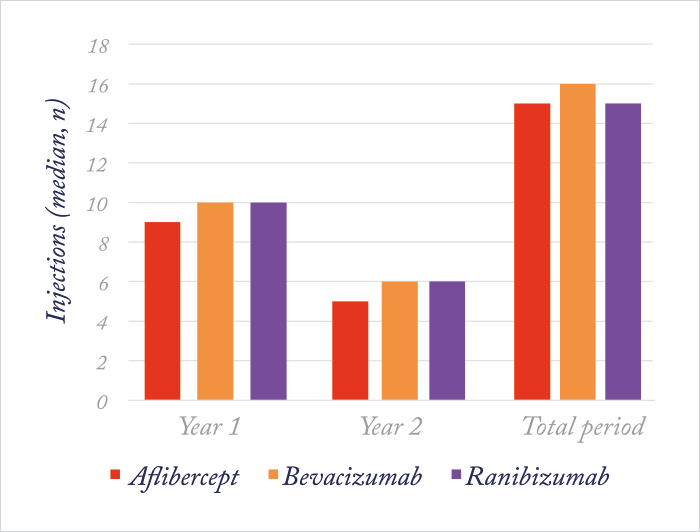
Although Protocol T’s primary outcome measure was assessed at 52 weeks (on the logic that if a difference was going to be seen in the relative efficacies of these drugs for the treatment of DME, it would be apparent by one year), the trial included follow-up through to the end of the second year of treatment. So when the results were presented at the 2016 Macula Society meeting (and published in Ophthalmology (2) almost immediately afterwards) there was considerable interest in whether or not these results held for the second year. In essence, it was a similar story after two years, but aflibercept lost its superiority over ranibizumab in the low-vision group (see Figure 2b) – and all three drugs continued to yield similar gains in vision from baseline. In patients treated with aflibercept, mean VA improved by 12.8 letters; in those who received bevacizumab, the improvement from baseline was 10 letters, and those in the ranibizumab group experienced an improvement of 12.8 letters. In those with a baseline VA of <69 letters (Snellen 20/50 or worse), mean VA improved by 18.3, 13.3 and 16.1 letters in the aflibercept, bevacizumab and ranibizumab groups, respectively. In patients with baseline VA of 78 to 69 letters (Snellen 20/32 to 20/40), mean VA improved by 7.8, 6.8 and 8.6 letters, respectively. Notably, focal/grid laser coagulation was given to 41 percent of patients in the aflibercept group, whereas 64 percent of patients in the bevacizumab group and 52 percent of patients in the ranibizumab group received it. All of this came with fewer injections in the second year (Figure 3) – almost half as many were administered to patients in the second year of the study as compared with the first.
On assessing adverse events, risk of heart attack, stroke, or death from a cardiovascular condition or an unknown cause by end of the trial was found to be higher among participants in the ranibizumab group. Twelve percent of ranibizumab participants had at least one event, compared with five percent of participants in the aflibercept group and eight percent in the bevacizumab group. Interestingly, this difference in cardiovascular event rates has not been seen across other studies, and therefore may be due to chance. Certainly, serious cardiovascular events are a known potential consequence of diabetes, but continued assessment of their association with these drugs looks like it’s going to be important in future studies. The occurrence of eye complications, such as eye infections and inflammation, was similar for all three drugs. The lead author of the Protocol T study, John Wells noted, “The results of the DRCR Network’s comparison of Eylea, Avastin, and Lucentis will help doctors and their patients with diabetic macular edema choose the most appropriate therapy,” adding, “The study suggests there is little advantage of choosing Eylea or Lucentis over Avastin when a patient’s loss of visual acuity from macular edema is mild, meaning a visual acuity of 20/40 or better. However, patients with 20/50 or worse vision loss may benefit from Eylea, which over the course of the two-year study outperformed Lucentis and Avastin.” These results need to be placed in the context of the trial’s location (the US) and the sponsors – the NEI (essentially the US federal government). Based on Medicare allowable charges, the per-injection costs of each drug (at the doses used in the study) were about $1,850 for aflibercept, about $60 for bevacizumab, and about $1,200 for ranibizumab. It’ll be interesting to see what difference the two-year results from Protocol T will make on anti-VEGF agent prescribing patterns in DME in the US going forward.
References
- JA Wells et al. “Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema”, N Engl J Med, 372, 1193–1203 (2015). PMID: 25692915. JA Wells et al. “Aflibercept, Bevacizumab, or ranibizumab for diabetic macular edema. Two-year results from a comparative effectiveness randomized clinical trial”, Ophthalmology (2016). Article in press.
