This article is an excerpt from a video initiative with Drs. Paulo E. Stanga, consultant ophthalmologist and vitreoretinal surgeon at the London Vision Clinic, London, UK; Hakan Kaymak, retinal specialist at Internationale Innovative Ophthalmochirurgie in Düsseldorf, Germany; and Carlos Orduna, retinal specialist at Oftalmologia Orduna in Madrid, Spain. Please click here to view the video series titled “Valeda Photobiomodulation Treatment for Dry AMD and Other Indications.”
The Valeda Light Delivery System is the first approved photobiomodulation (PBM)-based treatment for dry age related macular degeneration (AMD). PBM – also known as low-level light therapy – uses light exposure to stimulate cellular function, leading to beneficial clinical effects. By using a multiwavelength approach – 590 nm, 660 nm and 850 nm – PBM targets multiple cellular outputs to improve clinical outcomes. PBM has shown in clinical trials to improve vision and reduce drusen in dry AMD patients. The LIGHTSITE I clinical trial demonstrated positive effects of Valeda on best-corrected visual acuity improvements by five or more letters in 50 percent of PBM treated eyes (1). Additional clinical benefits shown in the trial included improved contrast sensitivity and reduced central drusen volume.
Chapter 1: Case studies of dry AMD patients treated with PBM using Valeda Light Delivery System.
Valeda Use in Clinical Practice
Dr. Hakan Kaymak runs a clinical practice that administers approximately 6,500 intravitreal injections on an annual basis. Dr. Kaymak has a great deal of experience using PBM for patients at both early and late stages of dry AMD and has seen excellent results, with functional vision improvements and reduction or resolution of drusen. Dr. Kaymak has also seen a benefit in using PBM for diabetic patients with early maculopathy, with a minimum of inner retinal fluid and good visual function.
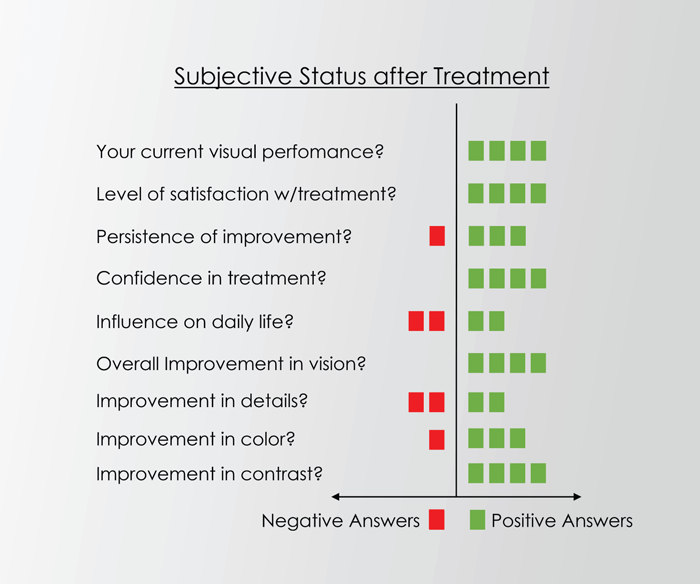
Dr. Kaymak notes that PBM is superior to the nano laser treatments he has used for many years, not only in terms of much improved function of the retina but also patients’ quality of life (see Figure 1). He begins the therapy with approximately nine treatments over three to four weeks and evaluates the results after four and 12 weeks. For positive long-term results, he recommends repeating treatment every three to six months, with some patients’ visual function being stable for as long as nine months.
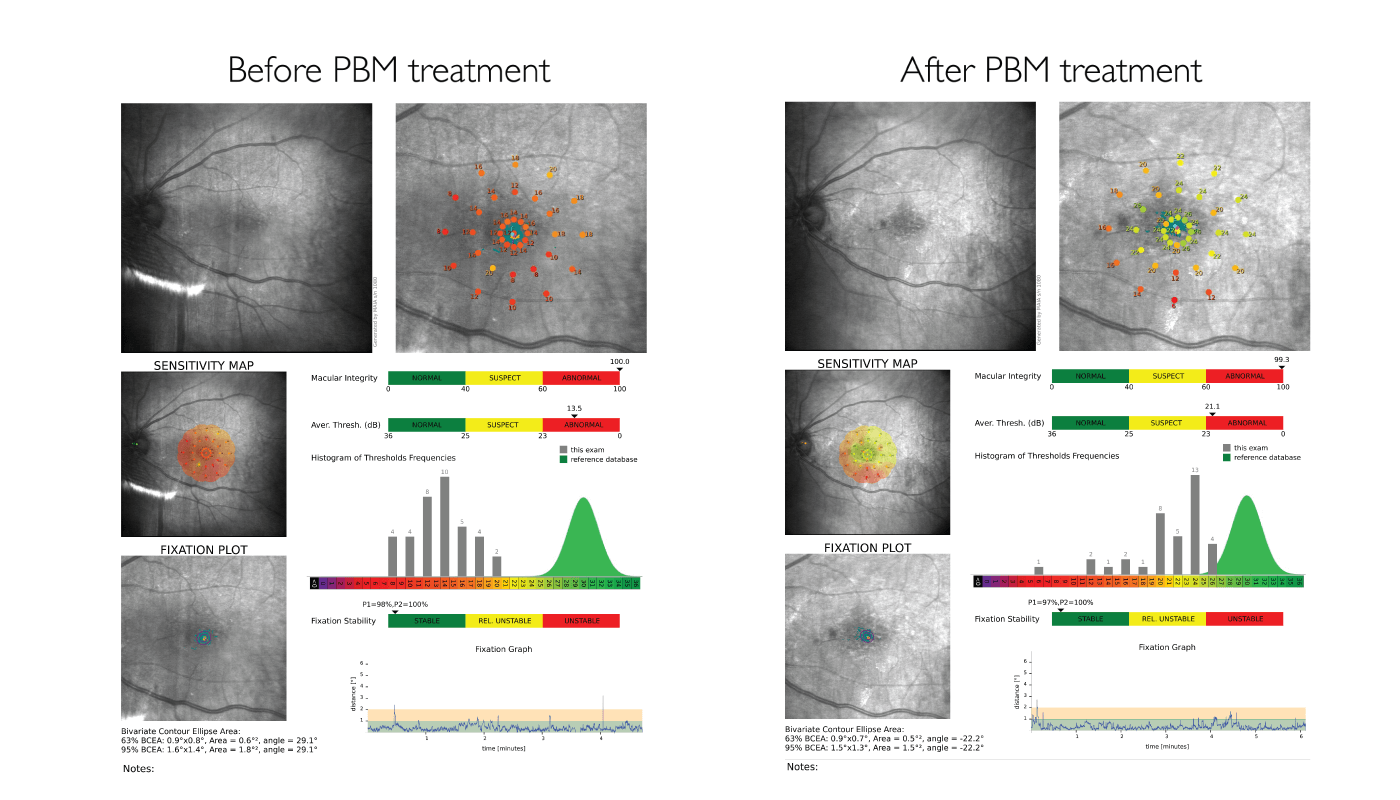
Dr. Carlos Orduna uses PBM for patients who have yet to reach the advanced stages of dry AMD but who are showing increasing drusen over a period of six months. Between November 2019 and June 2020 his practice used PBM for 105 patients, with 47 patients treated for dry AMD. Dr. Orduna saw a minimum improvement of three letters and maximum improvement as high as 22 letters; with 5–7 letters’ improvement seen in most patients. Dr. Orduna notes the importance of obtaining good microperimetry and recommends PBM for patients with small, hard drusen (see Figure 2). Although objectively measured results can vary, his patients often notice improved visual function, which boosts their quality of life.
Chapter 2: Patient selection and safety aspects of the new approach to retinal disease.
According to Dr. Orduna, patients with a clinical history of dry AMD, but with healthy retinal pigment epithelium, are the best candidates for this therapy. Dr. Kaymak offers PBM to all relevant patients, but he notes patients must be open to new treatment modalities. He believes it is important to communicate with patients in the right way – including highlighting treatment indications and the mechanism of action.
With many successful cases behind them, Drs. Kaymak and Orduna both view PBM as a safe treatment with no side effects that can improve visual acuity, contrast sensitivity, and patient quality of life.
Chapter 3: Valeda photobiomodulation treatment - patient cases.
References
- SN Markowitz et al., “A double-masked, randomized, sham-controlled, single-center study with Photobiomodulation for the treatment of dry age-related macular degeneration,” Retina, 40, 1471 (2020). PMID: 31404033.
