Like all treatments, anti-VEGFs have their pros and cons. Whilst effective for many patients with age-related macular degeneration (AMD) or macular edema (ME), frequent injections are needed and some patients can experience suboptimal outcomes. That’s exactly why the hunt is on for new and improved anti-angiogenic agents.
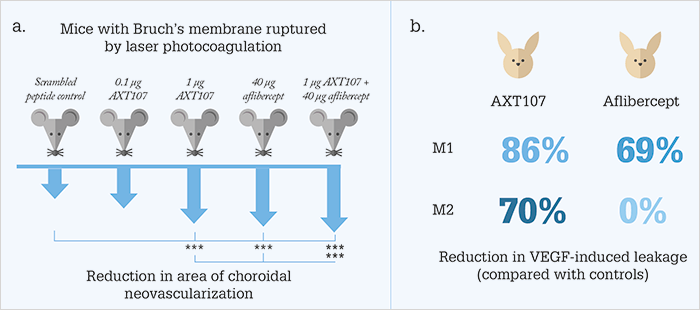
Joining the search are a group of researchers based at Johns Hopkins University School of Medicine, Baltimore, USA, who might be onto something with their biomimetic peptide derived from collagen IV. “Using bioinformatics, we identified shared sequences of proteins that have anti-angiogenic activity, and selected a series of peptides to test and optimize using cultured cells from blood vessels. The AXT107 peptide showed the most promise so we decided to investigate if it has the potential to treat disease,” says Peter Campochiaro, corresponding author on the paper (1). Comparing AXT107 treatment with aflibercept (and scrambled controls) in different animal models of retinal disease, the investigators saw promising results following injection of the peptide (Figure 1). “AXT107 suppressed abnormal blood vessel growth and leakage in several mouse models relevant to wet AMD and diabetic retinopathy, and showed similar efficacy to aflibercept,” says Campochiaro, adding “the combination of AXT107 and aflibercept was better than either alone.”
The team also encountered a surprising finding: following injection into the vitreous of rabbit eyes, the peptide formed a gel-like depot that could still be observed in the same location 30 days later. “The depot disassembled slowly, providing sustained delivery,” comments Campochiaro. “AXT107 suppressed abnormal vascular leakage for two months while aflibercept suppressed leakage for one month” (Figure 1b). With the belief that their findings will improve the treatment of patients with wet AMD, diabetic retinopathy, and retinal vein occlusion, Campochiaro indicates “These studies suggest that AXT107 may provide benefit for patients who are having suboptimal outcomes with current treatments, and may also reduce the frequency of intraocular injections that are needed.” Confirming that the team have had a pre-Investigational New Drug (IND) meeting, Campochiaro reveals that the team are currently performing the extensive toxicity studies that are needed before human trials can begin, which they anticipate will start before the end of the year.
References
- R Lima e Silva et al., “Tyrosine kinase blocking collagen IV–derived peptide suppresses ocular neovascularization and vascular leakage”, Sci Transl Med, 9 (2017). PMID: 28100891.
