- Worldwide, there’s a donor cornea shortage – but technical and handling reasons mean most surgeons won’t use a DMEK donor cornea younger than 40 years
- PDEK is a DMEK-like procedure that offers DMEK-like outcomes – but can use younger corneas – even those of infants
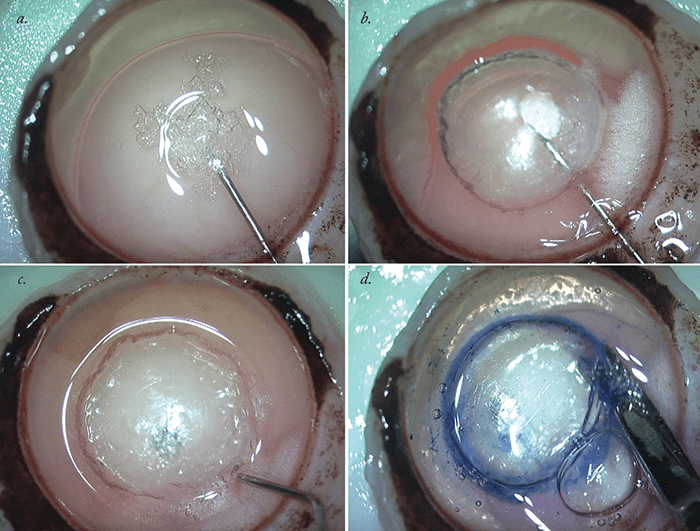
- It exploits the presence of the pre-Descemet (“Dua’s”) layer and uses a Type 1 big bubble to separate the PDL and the DM-endothelium complex from the residual donor stroma
- The younger tissue used in PDEK also has the advantage of increased endothelial cell density – meaning longer graft survival times
For the last few years, when it comes to treating corneal endothelial disorders with endothelial keratoplasty (EK), the debate has mostly been about whether to perform Descemet stripping endothelial keratoplasty (DSEK) or Descemet membrane endothelial keratoplasty (DMEK) (or their automated counterparts, DMAEK and DSAEK). The consensus is that DMEK is the more technically challenging of the procedures – but it does provide better visual outcomes. Rather than replacing the diseased DM-endothelium complex with posterior stroma (as with DSEK), you’re replacing it with healthy donor DM-endothelium (1)(2). However, the DMEK graft from young donors is more elastic, flexible, and difficult to harvest – and it tends to curl up slightly, making it difficult to unfold and position once inside the recipient’s eye. This along with the fact that it has a greater corneal curvature, means that most surgeons prefer to use donor corneas from those aged 40 years or older.
In 2013, Harminder Dua was the first to describe a pre-Descemet’s layer (PDL) of collagen, present between the corneal stroma and DM (3), and we have used this knowledge to produce a new EK variant: pre-Descemet membrane endothelial keratoplasty (PDEK). PDEK involves the separation of the PDL and the DM-endothelium complex from the residual donor stroma by the formation of a Type 1 big bubble (bb; (3)(4) – see Figure 1). The presence of the PDL makes the injection and bubble creation essentially as easy to perform as regular DMEK and should help avoid accidentally creating a Type 2 bb (5). PDEK has a number of advantages over previous EK methods: not only does it permit the use of younger tissue (dramatically increasing the potential donor pool), it also yields tissue that typically has a far greater endothelial cell density than typical DMEK grafts. The result should be a faster resolution of post-procedural corneal edema and improved graft longevity relative to DMEK-prepared grafts (4)(5). So how is it performed?
Donor graft preparation
The most important part of the PDEK procedure is preparation of the donor graft – and it’s essential to get it exactly right. The formation of Type 1 bb is essential for success (Figure 1) and separates the PDL from the DM-endothelium complex. This is in contrast with DMEK, which involves the formation of Type 2 bb via the passage of air in between the PDL and the DM-endothelial complex, leading to only the DM-endothelial layer being harvested, with the PDL being left behind in the remains of the donor graft. A mixed Type 1 and Type 2 bb can often be created during the air dissection procedure – in which case, the surgeon needs to be very careful to avoid any rupture of the bubble, while ensuring that the bb is passed along the correct plane. Harvesting a Type 2 graft would necessitate abandonment of PDEK graft preparation and the conversion of the graft preparation and surgical procedure to a DMEK procedure instead. Next, the donor graft (with corneo-scleral rim) is held endothelial side up. A 30 G needle attached to an air-filled 5 mL syringe is introduced from the periphery of the graft, into the center, and air is injected. A Type 1 bubble is formed that should spread from the center to periphery and should be around 8 mm in diameter. The edge of the bb is entered with a side port blade and Trypan Blue is injected inside to stain the graft. The graft is then cut with the corneo-scleral scissors all around the periphery of the bubble and it is then placed in the storage media.Mastering PDEK in 15 steps
- Step 1 – Fix the trocar anterior chamber (AC) maintainer (T-ACM). A routine anterior chamber maintainer can also be fixed if the surgeon is not well versed with the use of a T-ACM. Preparing an infusion set-up allows the surgeon to easily switch over between an air/fluid infusion as and when required during the surgical procedure.
- Step 2 – Connect T-ACM to an air pump. This facilitates continuous air infusion into the eye; it helps to perform the procedure with an AC that is always well formed.
- Step 3 – Two side port incisions are made at superotemporal and superonasal positions, to allow these sites to be utilized later in the procedure for further intraocular manipulation.
- Step 4 – The Descemetorhexis is performed with a reverse Sinskey hook – this step is essentially the same as in a DMEK procedure.
- Step 5 – A 2.8 mm clear corneal incision is made and the scored DM-endothelium complex is removed. This allows the introduction of the graft in to
the eye. - Step 6 – Inferior iridectomy is performed with a vitrector, and helps to prevent pupillary blockage from occurring.
- Step 7 – Load the PDEK graft in the cartridge of the foldable IOL applicator (with the injector spring removed), as originally described by Francis Price.
- Step 8 – Switch OFF the air pump. This step is performed before the graft is injected so that the force of the air does not displace the graft. It also ensures that there is enough room for the graft to enter and be properly placed in to the AC.
- Step 9 – Inject the PDEK graft in the AC. If the AC is shallow, then the assistant can inject some fluid in to AC from a side port incision.
- Step 10 – Suture the clear corneal incision; this ensures there is no wound leakage and that the graft is placed in a well-formed AC.
- Step 11 – The correct orientation of the graft is checked; an endo-illuminator can be used to facilitate this.
- Step 12 – Unroll the graft and inject a little air beneath it.
- Step 13 – Switch ON the air pump connected to the T-ACM. This pushes the graft and helps it adhere to the corneal surface.
- Step 14 – Unroll the graft fully using the reverse Sinskey hook and center it properly. Corneal massage is performed to adjust the centered position of the donor graft and to eliminate residual fluid at the donor graft–recipient interface. Residual interface fluid can also be drained through corneal venting incisions.
- Step 15 – Suture the wounds and remove the T-ACM.
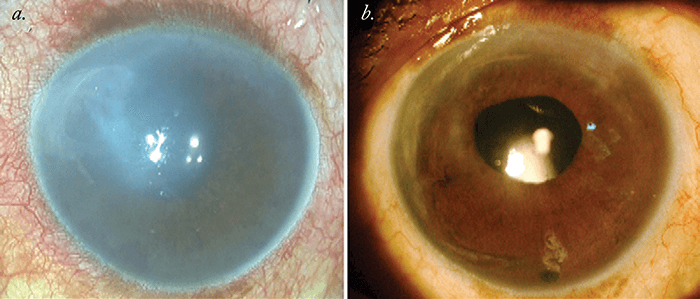
After completion of the surgical procedure, the patient is made to lie in the supine position for around two hours and is allowed only minimal movements for the next 24 hours. A postoperative regimen comprised of antibiotics and steroid drops is prescribed, and these are slowly tapered off over a period of three months, when corneal haze should have cleared (Figure 2).
Treating more with the same resources
The upper age limit for donor tissue usage is generally considered to be around 75 years – but there is still no clear consensus on the lower age limit of donor tissue. Most centers accept tissue from pediatric donors aged over 6 months – but we know that infant donor tissue has not been used because of fears associated with the technical challenges of preparing the graft and implanting such immature tissue. Indeed, when it comes to DMEK, it’s rare that anyone uses tissue from donors younger than 40 years of age partly due to the fear of tearing the DM during donor tissue harvesting, due to the strong adhesion between the DM and stroma. PDEK obviates this risk and, in a world where many countries face a shortage of donors, it enables a far greater pool of tissue to be used to treat corneal endothelial disorders with DMEK-quality outcomes. Amar Agarwal is Chairman, Dr. Agarwal’s Group of Eye Hospitals, Chennai, and a pioneer of many techniques and procedures used routinely today in corneal and cataract surgery.References
- GR Melles et al., “A surgical technique for posterior lamellar keratoplasty”, Cornea, 17, 618–626 (1998). PMID: 9820943. GR Melles et al., “Descemet mem¬brane endothelial keratoplasty (DMEK)”, Cornea, 25, 987–990 (2006). PMID: 17102683. HS Dua et al., “Human corneal anatomy redefined: A novel pre-Descemet’s layer (Dua’s layer)”, Ophthalmology, 1778–1785 (2013). PMID: 23714320. A Agarwal et al., “Pre-Descemet’s endothelial keratoplasty (PDEK)”, Br J Ophthalmol, 98, 1181–1185 (2014). PMID: 24659352. A Agarwal et al., “Pre-Descemet Endothelial Keratoplasty with infant donor corneas: a prospective analysis”, Cornea, 34, 859-865 (2015). PMID: 26057329.
