- Recent years have seen an influx in diagnostic and treatment regimens for ocular surface disease (OSD), with matrix metalloproteinases (MMPs) and osmolarity becoming increasingly important
- MMPs are proteolytic enzymes produced by stressed epithelial cells on the ocular surface – and they destabilize the tear film
- Identifying elevated MMP-9, which indicates inflammation, helps guide therapeutic decision making. For example, elevated MMP-9 may predict the patients most likely to respond to anti-inflammatory therapy
- Incorporating these elements into your OSD algorithm can determine the severity of dry eye disease and dictate treatment, all while adhering to TFOS DEWS II, MGD Workshop, and CEDARS recommendations.
A groundswell of interest in the diagnosis and treatment of ocular surface disease (ODS) is reflected in the current array of algorithms aimed at simplifying the approach. These stepwise diagnostic and therapeutic recommendations seek to coalesce around the most recent clinical data and practical experience with consensus and clarity. Successful refractive cataract surgery hinges on the health of the corneal surface, which allows accurate and repeatable preoperative measurements, particularly with respect to biometry and keratometry. Precise data is crucial to guide the best IOL selection for the patient and ultimately, to ensure his or her overall satisfaction with the visual outcomes – driving ophthalmologists to present and hone OSD diagnostic and treatment regimens.
My personal decision-making process relies on the cornerstone of our understanding of OSD: hyperosmolarity is the central mechanism that triggers the cycle of inflammation that ultimately leads to epithelial cell damage and/or death. This loss of homeostasis causes the tear film to become unstable, with commonly associated symptoms of discomfort and/or visual disturbance [see box] (1, 2).
In dry eye disease (DED), hyperosmolarity is considered to set up a cascade of signaling events in surface epithelial cells that leads to the release of inflammatory mediators and proteases. Such mediators, together with the hyperosmolarity itself, cause goblet and epithelial cell loss and damage to the epithelial glycocalyx.
Damage is reinforced by inflammatory mediators from activated T-cells, recruited to the ocular surface. The net result is the characteristic punctate epitheliopathy of DED and tear film instability, which leads to early tear film break-up. This exacerbates and amplifies hyperosmolarity and completes the vicious circle events that produce ocular surface damage.
Supported by peer-reviewed literature, data collection, my years of experience, in addition to recently published algorithms like TFOS/DEWS II (2), the MGD Workshop (3) and CEDARS Dysfunctional Tear Syndrome (4), my approach has remained largely unchanged for the past eight years. By using tear osmolarity as a base from which to build, I have developed clear-cut guidelines for initiating treatment in my OSD patients – and tracking their response.
Although the concept of osmolarity is no newcomer to dry eye disease’s definition, it has become increasingly important from a clinical point of view; its utility in diagnosing and monitoring dry eye disease has been repeatedly highlighted (5). TearLab’s Osmolarity System measures the osmolarity of tears with easy-to-interpret data, making the results informative whether normal or abnormal. The system’s Osmolarity Test Card provides a quick and simple method for determining tear osmolarity using nanoliter (nL) volumes of tear fluid collected directly from the eyelid margin. The TearLab Test Card is held by the Osmolarity Pen, for safe collection. An elevated reading of 300 or above or an intra eye difference of 8 mOsm/L or more indicates instability of the tear film (1, 2).
- Normal: Under 300 mOsm/L
- Abnormal: 300 mOsm/L or higher
- Mild: 300-320 mOsm/L
- Moderate:320-340 mOsm/L
- Severe: 340 mOsm/L or higher
- Instability in tear film: intra eye difference of 8 mOsm/L or more
Matrix metalloproteinases (MMPs) are proteolytic enzymes produced by stressed epithelial cells on the ocular surface. MMP-9 destabilizes the tear film and directly contributes to corneal barrier dysfunction by breaking down tight junctions and facilitating inflammatory cell migration. This mechanism negatively impacts corneal integrity, signaled by elevated MMP-9 in the tears, correlating with examination findings in moderate to severe dry eye (1, 5, 6, 7, 8).
Note that not all dry eye patients have clinically significant inflammation, and the traditional dry testing methods, such as tear breakup time, Schirmer, and osmolarity, cannot predict the patients who do. Identifying elevated MMP-9 – an indication of inflammation – helps guide therapeutic decision making. For example, elevated MMP-9 may predict which patients are most likely to respond to anti-inflammatory therapy. The down regulation of MMP-9 expression is associated with improvement in ocular surface epithelial cells; therapy with artificial tear products alone fails to demonstrate a reduction in MMP-9 levels (9).
InflammaDry (Quidel) tests a tear sample taken from the patient’s palpebral conjunctiva. The sample collector is inserted into the test cassette. The results are ready in 10 minutes, red plus blue equals a positive result and blue equals negative (10). InflammaDry should be performed before instilling ocular anesthetic, topical dyes or performing Schirmer testing.
Normal levels of MMP-9 in human tears range from 3 to 41 ng/mL;
- a positive test = MMP-9 ≥ 40 ng/mL
- a negative test = MMP-9 < 40 ng/mL
When MMP-9 is positive, the patient has inflammation (9), which should be treated preoperatively for an optimal result. When tear osmolarity testing and MMP-9 are both positive, DED can be confirmed as the cause of inflammation. In either situation, positive MMP alone or with positive osmolarity, inflammatory DED will respond to anti-inflammatory treatment. Negative osmolarity plus positive MMP-9 indicates a non-dry eye cause of inflammation, such as allergic conjunctivitis, which requires a closer look as well as appropriate treatment.
Patients receive a psychometric questionnaire upon arrival; if there is even one positive response, our technicians can test for tear osmolarity and MMP-9 before the doctor sees the patient. Any patient who has at least one positive response on the questionnaire, a tear osmolarity test of 317 or higher, and/or a positive MMP-9 test, has their preoperative cataract evaluation abbreviated. At this stage, the surgeon meets the patient, performs a short slit-lamp examination and has a brief conversation to explain the need to treat DED before surgery so that the cataract evaluation will be accurate and that there will be more IOL options available.
The vast majority of DED patients (approximately 85–90 percent in a typical cataract practice, I would estimate) need just three things at the end of the shortened cataract evaluation:
- lifitegrast 5 percent two drops, twice a day
- gently preserved or preservative free artificial tears four times a day
- omega-3 supplements
We schedule the patient to return for their cataract evaluation in two to three weeks, and that is when the discussion takes place about the specific type of surgery and IOL choices. In my experience, patients are grateful for the meticulous care and caution; you will not lose patients. Any cataract surgeon can incorporate this approach to OSD into their flow, even if he or she does not wish to concentrate on dry eye.
But what if the patient has a more advanced level of OSD, gets worse, or needs more treatment? Consider treating more advanced OSD, which is rewarding, lucrative and will not cannibalize your cataract practice. Alternatively, pass the patient onto a colleague, an ophthalmologist or optometrist, from inside or outside of the practice, who can act as the OSD consultant. A trusted a dry eye expert will treat your OSD patients and return them to you for surgery.
Here is a closer look at how I use tear osmolarity to guide my treatment, which starts at 296 mOsm/L or higher with gently preserved or preservative-free tears four times a day and omega 3s.
- 317 mOsm/L, lifitegrast twice a day (cyclosporine can also be used if there are no time constraints, as it takes three to six months to take full effect)
- 325 mOsm/L, switch to preservative-free artificial tears eight times per day plus bland ointment every night at bedtime (add plugs at next visit if osmolarity still above 325 mOsm/L)
- At 330 mOsm/L, order blood test for Sjogren syndrome and switch to autologous serum tears eight times a day, and add the other prescription medication (i.e., if they are already on lifitegrast, add cyclosporine, and vice versa)
- At 335 mOsm/L, consider contact lenses made from amniotic membrane, e.g., the Prokera Slim (BioTissue) for five days, one eye at a time
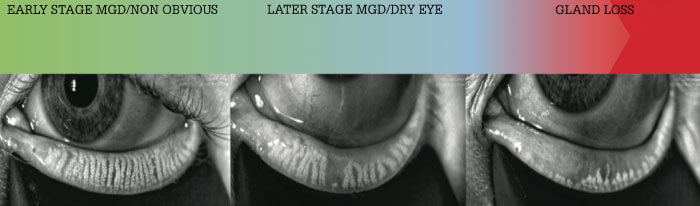
Nichols and Lemp have estimated that 86–92 percent of dry eye patients have an evaporative component of their OSD caused by meibomian gland disease (MGD) (11, 12), making meibography an integral device. This powerful tool both confirms the diagnosis and importantly encourages compliance with the suggested treatment regimen, motivating patients to perform preventive care.
Using meibomian gland imaging, I look for duct dilation, gland constipation, curling and shortening (atrophy), hazy appearance, and dropout. Once only used in research settings, today’s systems make it easy to evaluate the structure of the glands, helping to also identify non-obvious MGD. I use an MGD grading system (Figure 1) that closely mirrors the MGD Workshop recommendations (3) to inform my decision to add more treatments to the osmolarity and MMP 9-based treatment algorithm. At any severity level, I suggest introducing thermal pulsation therapy for patients doing the recommended regimen but who are still symptomatic, and/or patients who struggle to adhere to – or truly hate – the recommended routine below
The decisions are based on my grading of MGD at the slit lamp and meibography:
- At stage one or less (very mild MGD), I observe (note, patient is already on tears and omega 3s)
- At stage two (mild to moderate MGD), I add soaks and scrubs twice daily and switch the bland ointment to erythromycin or bacitracin ointment every night at bedtime. I encourage microblepharoexfoliation (see box Microblepharoexfoliation) with the BlephEx unit, two to four times a year, and thermal pulsation therapy once a year. Alternatively, intense pulsed light or IPL is also gaining popularity for MGD (13).
- At stage three (moderate to severe MGD) – or for any patient with a history of recent chalazion – I add doxycycline 50 mg by mouth daily for one or more months. (Note: Ask about pregnancy and warn patients of photosensitivity. Patients should not take on an empty stomach but avoid within an hour of dairy consumption.)
- At stage four (severe MGD), I recommend monthly in-office lid cleansing treatments with disposable, single-use eyelid cleansing sticks from OCuSOFT (Figure 2) – in addition to all previous therapies, including microblepharoexfoliation two to four times a year, and thermal pulsation therapy once a year.
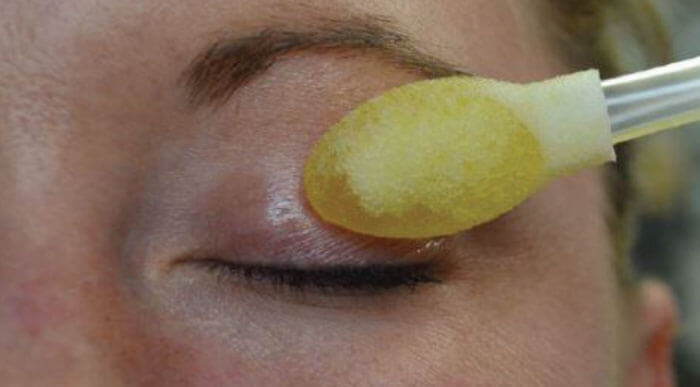
- Spins a micro-sponge 2,500 RPM along margin to remove the biofilm
- Results in completely clean lids
- Repeated every 3-6 months
- Private pay procedure

I have structured my own OSD algorithm around tear osmolarity and MMP-9 as the primary elements. This algorithm adheres closely to the TFOS DEWS II, MGD Workshop, and CEDARS recommendations. These two quick tests help in determining the severity of DED and dictate treatment. Lid disease therapies are added as needed (as a majority of DED patients have concomitant MGD).
References
- M Lemp et al., “Tear osmolarity in the diagnosis and management of dry eye disease”, Am J Ophthalmol, 151, 792 (2011). PMID: 21310379.
- J Craig et al., “TFOS DEWS II Report Executive Summary”, Ocul Surf, 15, 802 (2017). PMID: 28797892.
- J Nelson et al., “The International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee”, Invest Ophthalmol Vis Sci, 52, 1930 (2011). PMID: 21450914.
- M Milner et al., “Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment”, Curr Opin Ophthalmol, 28, 3 (2017). PMID: 28099212.
- B Sullivan et al., “An objective approach to dry eye disease severity”, Invest Ophthalmol Vis Sci, 51, 6125 (2010). PMID: 20631232.
- R Sambursky et al., “Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease”, Cornea, 33, 812 (2014). PMID: 24977985.
- R Potvin et al., “Tear film osmolarity and dry eye disease: a review of the literature”, Clin Ophthalmol, 9, 2039 (2015). PMID: 26586933.
- R Sambursky et al., “MMP-9 and the perioperative management of LASIK surgery”, Curr Opin Ophthalmol, 22, 294 (2011). PMID: 21537181.
- R Sambursky, “Presence or absence of ocular surface inflammation directs clinical and therapeutic management of dry eye”, Clin Ophthalmol, 10, 2337 (2016). PMID: 27920494.
- RPS InflammaDry sensitivity and specificity was compared to clinical truth in RPS clinical study: protocol #100310.
- M Lemp et al., “Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study”, Cornea, 31, 472 (2012). PMID: 22378109.
- K Nichols et al., “The International Workshop on Meibomian Gland Dysfunction: Executive Summary”, Invest Ophthalmol Vis Sci, 52, 1922 (2011). PMID: 21450913.
- S Dell et al., “Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction”, Clin Ophthalmol, 11, 817 (2017). PMID: 28496300.
