
- Ophthalmic pathology might be one of the less common ophthalmic specialisms, but it is no less crucial
- Here, two leaders in the field share their career journeys and overview their day-to-day work
- Sarah Coupland shares what inspired her to enter the field of ophthalmic pathology, and provides an insight into her work on ocular oncology
- Hans Grossniklaus discusses balancing his pathology and research roles with being an ocular oncologist, and shares what is exciting in the field right now.
Director of the North West Cancer Research (NWCR) Centre, Liverpool; Professor and George Holt Chair of Pathology at the University of Liverpool and Honorary Consultant Histopathologist at Royal Liverpool and Broadgreen University Hospitals NHS Trust, UK; and former Vice-President of ARVO. Specialism: Ocular oncology Key inspiration: “I have always had a fascination about human biology and physiology, and understanding the mechanisms behind them. Pathology is essentially the understanding of what alterations occur in these processes to initiate disease, and enables a methodological and scientific approach to better diagnose and treat the conditions.” Notable memories: “Recently receiving the International Council of Ophthalmology (ICO) Ophthalmic Pathology award at the World Ophthalmology Congress (WOC) in Barcelona, Spain. This award is only given out every four years, and it is a huge honor to receive it.”
What inspired you to enter ophthalmic pathology?
My father was a medical oncologist and my mother a nurse, so I grew up with “medical speak” over the dinner table – it almost became second nature to me. I always wanted to study medicine, and after graduating from Medicine in Sydney, I moved to Berlin, and began a PhD in ophthalmology – I was interested in specializing in this field because of its fine surgery. My PhD examined the immune mechanisms involved in corneal rejection and how these could be influenced by various immunosuppressive drugs. I performed corneal transplantations in rats, followed by histological and immunohistological examination of their eyes. And that’s how I rediscovered my enthusiasm for the morphological understanding of disease mechanisms, and how they could be modified by treatment. After completing my PhD, I did a three-month elective with William Lee in Glasgow – a period during which I finally made the decision to specialize in histopathology. I then spent seven years training in general pathology with Harald Stein at the Charité Benjamin Franklin, Berlin – at that time a referral center for lymphomas, head and neck surgery and ophthalmic tumors – and emerged with a number of pathology subspecialties under my belt.What is unique about ophthalmic pathology?
As an ophthalmic pathologist concentrating on ocular oncology, I interact closely with clinical teams. Ophthalmological diagnoses are very reliant on morphology and images. The beauty of the eye – and the surrounding structures – allows both the ophthalmologist and the pathologist to literally see many disease processes ‘in situ’ in the patient, which can allow for easier interpretation of the samples. That being said, many cases are difficult because the samples are tiny! For example, intraocular biopsies of the choroid or vitreous can be very demanding; one is expected to squeeze out as much information as possible on morphology, immunophenotype and genotype. Ocular tumors are rare, but this does not mean that they are any less malignant (or less fatal) than the more common cancers. The majority of our work focuses on uveal melanoma – the most common primary intraocular tumor in adults – and we have a very good collaboration with the clinical team. Unfortunately, approximately 50 percent of these patients develop metastases to the liver, which at present are not curable. We offer a molecular prognostic service that helps classify uveal melanomas into those that have a good or poor prognosis based on chromosomal abnormalities and mutations in the tumor cells. That prognostic patient curve is used by the clinical team, as well as the clinical psychologists, to discuss the prognosis of the patient and the risks of developing metastases. Patients with tumors that show an intermediate to a high risk of developing metastases to the liver are then stratified by the clinicians and the medical oncologists for more intense liver surveillance. The algorithms for our prognosticator are built on data from over 2,000 patients and we’re constantly refining the tool as we obtain more data; we’re currently in the process of validating the third version via a multicenter collaborative study. My favorite aspect of ophthalmic pathology work is making a difficult diagnosis in a timely manner to improve a patient’s outcome. The typical scenario would be a vitreous biopsy for suspected vitreoretinal lymphoma. These are notorious for the fragility of the tumor cells and the relatively high rate of non-diagnostic samples. By working closely with the vitreoretinal surgeons, we have been able to make recommendations with respect to how the sample is taken, transported and processed in the lab to improve the diagnostic yield. Vitreoretinal lymphomas are high-grade tumors and so diagnostic delays must be avoided to improve the patient’s chance at survival.
What are you currently working on?
My main area of research is trying to understand uveal melanoma and improve the therapy of metastatic melanomas, with the ultimate aim of improving outcomes for patients – which at the moment are pretty abysmal. As I mentioned above, around half of uveal melanomas metastasize into the liver. As they locate to the space of Disse, an immune privileged site, there often isn’t an inflammatory response until the metastatic tumors are quite large and have really taken ‘root’ in the liver. Moreover, these tumor cells manipulate hepatic stellate cells to induce fibrosis – almost like they are creating their own fortifications. We’re currently investigating the interactions between uveal melanoma cells and hepatic stellate cells to understand the fibrotic process (1). We have also shown that cultured uveal melanoma cells can synthesize their own lattice-like extracellular matrix upon which they can grow (2). As this peri-tumoral and intra-tumoral fibrosis might hinder the access of drugs and inflammatory cells to the tumor cells, it is important that we understand the tumor microenvironment to develop effective strategies that actually allow therapies to reach the target cells. We’re also currently collaborating with 12 other groups on a project called UM Cure 2020, which is using genomics, proteomics and metabolomics technologies to analyze human uveal melanoma metastases to try and better understand the characteristics of the tumor cells and determine if we can use any new targeted therapies. There are also two pharmaceutical companies involved in the project who are looking for druggable targets. UM Cure 2020 is being led by Institut Curie in Paris, France, but as we are a major component as are coordinating the virtual biobank across the consortium and the histological examinations of a variety of specimens. In Liverpool we have created a unique oncology biobank with primary tissues and associated bloods from patients, allowing us to take part in projects such as UM Cure 2020 and The Cancer Genome Atlas (TCGA) (3).If you could change one aspect of your field, what would it be?
I was taught that the pillars in the understanding of medicine are the “three Ps”: pathology, physiology and pharmacology. If we are to make progress in the understanding of the pathogenesis, prevention and treatment of disease, we have to invest in these cornerstones of scientific medicine. Academic pathology is one of the most fragile subspecialties in medicine at present, pathologists have to come out of the shadows, and have to increase awareness of its importance and create initiatives to make it attractive and prevent its complete disappearance.Interim Vice Chair, Emory Eye Center; F. Phinizy Calhoun Jr. Professor of Ophthalmology; Director, L.F. Montgomery Pathology Laboratory; Director, Section of Ocular Oncology and Pathology; Emory Eye Center, Atlanta, GA, USA. Specialism: Ocular oncology and pathology Key inspiration: “My interest in ophthalmology was inspired by Torrence Makley and William Havener at Ohio State University. While a medical student, I read many articles by W. Richard Green at the Wilmer Eye Institute, and was impressed by his knowledge and productivity. As an ophthalmology resident, I attended the Armed Forces Institute of Pathology (AFIP) course on Ophthalmic Pathology, and was inspired by Lorenz Zimmerman, Dick Green, Ramon Font, Jerry Shields and others to pursue a career in ophthalmic pathology and oncology.” Notable memories: “Whilst there have been many notable experiences, I remember a case in which we were able to decipher a rare condition that had baffled the experts as X-linked lymphoproliferative disease. It was very rewarding to find a long sought after answer.”

As an ophthalmologist, pathologist and a researcher, you wear quite a few ‘hats’…
Yes – but I have combined all three so that my practice and my research are focused. My pathology practice is geared towards tumors, my clinical practice is as an ocular oncologist and my research is on ocular tumors, so it all gels. The key elements are working as a practicing ocular oncologist, ophthalmic pathologist, and ocular oncology translational scientist focused on melanoma and retinoblastoma. I am able to combine seeing patients and treating them (surgically, and/or with lasers and targeted medications) with an intimate understanding of the pathology of their disease. This, combined with research directed at improving my patients lives, are key elements to my roles. A “typical” day would be seeing patients or doing surgery during half of the day as a practicing ocular oncologist, signing out ophthalmic pathology cases as a diagnostic ophthalmic pathologist and designing/performing/evaluating experiments as a translational experimental pathologist. During all of this, I am also teaching medical students, residents and fellows.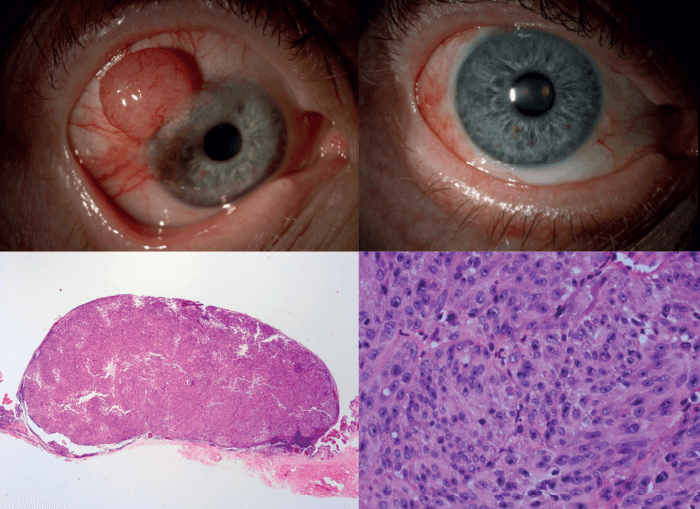
What is most exciting in the field, right now?
There are exciting new potential treatments for metastatic uveal melanoma. Although checkpoint inhibitors have proven to be relatively successful for skin melanoma and metastatic cutaneous melanoma, they haven’t worked so well for uveal melanoma and metastatic uveal melanoma. But combination strategies may help enhance efficacy. Though I don’t think we will be able to eliminate all metastatic melanoma, hopefully we can achieve tumor involution. It will be more like treating a chronic disease, which will prolong people’s lives. Our work in Ethiopia through the Emory Eye Center Global Health Initiative is is also very exciting. This project relates to access to retinoblastoma care. Unlike uveal melanoma where the primary tumor can be treated but people are still dying from metastatic disease, retinoblastoma can be treated quite successfully with a very high survival rate in the US and Europe (around 97–99 percent). But in other regions of the world, like Ethiopia, there is only around a 50 percent survival rate. Our team is working to improve medical care access for children in Ethiopia, so that their retinoblastoma can be treated at an earlier stage. When I think back, it was by serendipity that the project came together. I had two visiting Ethiopian clinicians in my lab, who asked what they could work on. I said we should work on retinoblastoma and the problems in Ethiopia. Just by happenstance, Jacquelyn O’Banion came to my lab and said that she’s working on retinoblastoma in Ethiopia and asked if I wanted to be involved. I said that’s exactly what I’m starting to work on! And it all came together. We recently had a symposium in Addis Ababa, which was organized by Fran Wu, and involved members of the Ethiopian government, providers (including the ophthalmologists who take care of the children with retinoblastoma), as well as medical oncologists and pathologists. From this we developed an action plan that is now being implemented, and we will follow up in a year or two to assess how far we have gone with these action items. There is a team involved in public awareness screening and referral, and we want to establish a diagnosis and pathology team, as well as treatment and follow-up team. We’re currently working on raising funding for the next meeting, and anyone who might be interested in being involved is welcome to contact me.What’s the most special aspect of your field?
We are a small international community of ocular oncologists and pathologists, and we try to work together to solve problems and take care of patients – it’s a true team approach. I feel fortunate and honored to take care of my patients – and to work with such wonderful and gifted ophthalmologists and pathologists.References
- I Ahmed et al. “The interaction of uveal melanoma (UM) with hepatic stellate cells (HSC)”. Presented at ARVO; April 28–May 3, 2018; Honolulu, HI, USA. S Coupland et al., “Liver fibrosis and metastatic uveal melanoma (mUM)”. Presented at ARVO; April 28–May 3, 2018; Honolulu, HI, USA. AG Roberston et al., Cancer Cell, 32, 204–220 (2014). PMID: 28810145.
