- Rod photoreceptors are ~40 percent more metabolically active when dark-adapted than when in the light – and that demand is met by increased perfusion of the retinal vasculature
- Diabetes damages the microvasculature – including that of the retina. Compromised circulation can lead to retinal hypoxia, VEGF expression, neovascularization and edema
- Nocturnal ocular illumination can prevent dark adaptation, and the Troxler effect means that patients rapidly stop “seeing” the light
- Reducing retinal oxygen demand should reduce hypoxia, and with it VEGF production – and potentially the number of anti-VEGF injections with it, with obvious socioeconomic benefits
The retina uses more oxygen per unit mass than any other tissue in the body, due to the fact that photoreceptors have a phenomenally high metabolic rate. That oxygen demand becomes even greater at night, rising by around 40 percent, as rod photoreceptors dark-adapt. Under normal physiologic circumstances, this isn’t a problem; the additional demand for oxygen is met by increased blood flow through the retinal vasculature. However, problems start to occur when that vasculature becomes damaged.
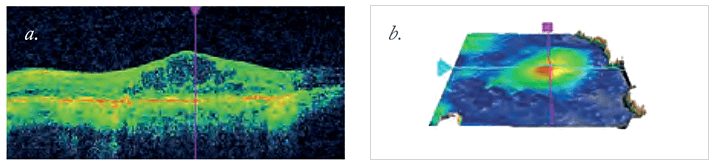
A growing body of research has found that diseases such as diabetic retinopathy (DR) and diabetic macular edema (DME) are driven, at least in part, by retinal hypoxia. People with diabetes commonly have microvascular damage, which can start to compromise retinal blood circulation. Once circulation is sufficiently compromised, the result is retinal hypoxia. This leads to upregulation of vascular endothelial growth factor – VEGF – with the consequence being retinal neovascularization. As those new vessels are leaky, the result is DME (Figure 1).
The current standard of care for DME treatment, depending on the degree of edema, is either laser photocoagulation or intravitreal injection of anti-VEGF drugs, typically bevacizumab, ranibizumab or aflibercept. The anti-VEGF agents are highly effective in the majority of cases, but even with pro re nata or treat-and-extend regimens, they still require patients to make regular hospital visits to receive their injections. For a limited population of patients, there’s also the option of steroid implants. Two are currently available: Allergan’s Ozurdex and Alimera’s Iluvien, both of which provide longer-lasting therapeutic action (of 6 and 36 months, respectively) than anti-VEGF agents, but are reserved for patients who, according to their summaries of product characteristics, are “insufficiently responsive” or “unsuitable for non-corticosteroid therapy.” That’s partly because intravitreal steroid use carries the risk of raised intraocular pressure, and the development of cataract in the phakic eye – so careful consideration is required before their use. The other factor is cost; these agents don’t come cheap. Diabetes is a huge economic burden on healthcare systems around the world. In the UK, where we’re based, diabetes costs our National Health Service (NHS) more than £10 billion each year – 10 percent of the total healthcare budget – and a large proportion of that cost is drugs to treat the ocular complications of diabetes. It’s only going to get worse as diabetes prevalence rises, potentially by as much as 50 percent by 2030 in the UK, with obvious pharmaco- and socioeconomic implications. Anything that could reduce the number of hospital visits, and particularly, anti-VEGF injections required, could make a huge difference to patients’ lives and healthcare costs.
Better than sleeping with the lights on
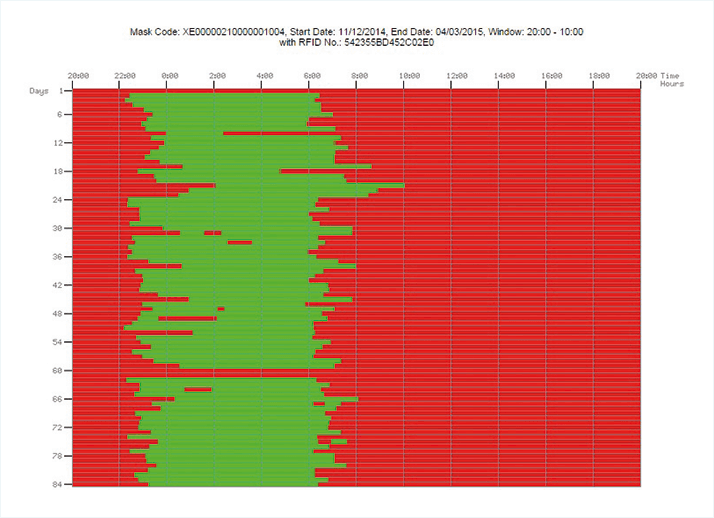
If at least part of the genesis of DR or DME is retinal hypoxia, and this can be caused by dark adaptation, then might preventing dark adaptation from occurring reduce the metabolic demands on the retina and alleviate the disease processes? Since humans only dark-adapt at night during sleep, sleeping in an illuminated environment should prevent or reverse the condition. The problem with sleeping with the lights on is that patients might sleep on one side, on their face, move under the blankets… and this doesn’t lend itself to rigorous scientific assessment or consistent clinical effects. The way around that is to create sleep masks that emit light to uniformly illuminate the eyelids during sleep. It’s important that the masks are comfortable to wear and do not disturb sleep; constant illumination should not be disruptive, as the eye adapts quickly due to the Troxler effect. Ultimately, the result of this thought process was the development of the Noctura 400 (Figure 2), developed by biophotonic research and development company PolyPhotonix. It contains a removable lighting pod that emits a mellow green light into closed eyes throughout the night while a patient sleeps. The light source is an organic light-emitting diode (OLED), which is tailored to emit precise wavelengths to suit the application and powered by an onboard coin cell battery that has a three-month life span. You may have heard of pill bottles that can track patients’ drug regimen compliance; Noctura 400 can do something similar. It has a compliance monitoring system that senses and records how long the light mask is worn, and provides nightly data on the amount of therapy being administered (Figure 3), meaning that clinicians can examine treatment adherence and link that to the condition of patients’ retinas.
Clinical validation
Developing the sleep mask involved a number of collaborations across the UK. The Northern Design Centre of Northumbria University helped determine the mask’s shape and appearance, and the School of Medicine, Pharmacy and Health at Durham University, provided valuable insight into patient issues. A close relationship between PolyPhotonix and the Eye and Vision Department at Liverpool University established the safest wavelengths and light intensity to use, and now a clinical trial has been completed demonstrating the safety and acceptability of the treatment. In addition, the Noctura mask has been evaluated by a Czech patient group in a six-month clinical trial that involved patients with advanced diabetic eye disease, with favorable results. It was the data from these trials that allowed the Noctura 400 to be awarded a CE mark.A large three-year randomized controlled clinical trial is underway, based at 15 hospital eye departments throughout the UK. The trial was designed to clarify whether mask wearing can effectively combat diabetic eye disease, and to identify which patients derive the most benefit. Three hundred patients were randomized to light masks or control masks, in order to determine whether the therapy can prevent development and progression of DME, or even lead to regression of the disease.
The implications
Today, the Noctura 400 sleep mask is currently being clinically evaluated in more than 35 NHS hospital clinics and has already recorded over 150,000 hours of use. The UK’s National Institute for Health and Care Excellence (NICE) has recognized the clinical potential of Noctura 400 for the treatment of patients with diabetic eye disease, and the mask is on course to be fast-tracked through the NICE evaluation process. NHS funding has also been made available to carry out evaluations in the community alongside the clinical trials, and a number of commercial pilots are underway; one with a number of optometrists in the North East of England and one national pilot with a group called The Outside Clinic, that visits patients’ houses and provides an optician service. Of course, the technology that underpins Noctura 400 may also benefit patients with other retinal degenerative diseases. PolyPhotonix is currently in the early production stages of the Noctura 500, a version designed for the treatment of wet age-related macular degeneration (AMD). This latest incarnation has been made available for its first pilot trial, to be conducted in centers in both Cardiff and Bristol. Today’s treatment of DME and wet AMD is invasive, costly, inconvenient, and hospital-based. The sleep mask is noninvasive, dramatically less expensive than chronic anti-VEGF treatment regimens, and will help allow patients to take charge of their own treatment at home, under the care of their physicians. If it lives up to the promise already shown in clinical trials, Noctura could profoundly change the future management of diabetic eye disease and wet AMD.Ian Grierson is Emeritus Professor of Ophthalmology, Liverpool University, Visiting Professor University of Cardiff, UK, and Special Trustee Moorfields Eye Hospital and Scientific and Medical Adviser to PolyPhotonix.
Richard Kirk is Chief Executive Officer of PolyPhotonix.
