Pegpleranib (E10030; Fovista) had the potential to be worth a billion dollars to Ophthotech. The rationale was obvious. Platelet-derived growth factor (PDGF) is the enemy of sustained anti-VEGF therapy: it causes pericytes to proliferate and engulf the new macular vasculature, thereby protecting the vessels against the effects of the anti-VEGF agents. Fovista blocks PDGF, disrupting the pericytes and stripping them from the blood vessels, rendering them vulnerable once again to anti-VEGF drugs.
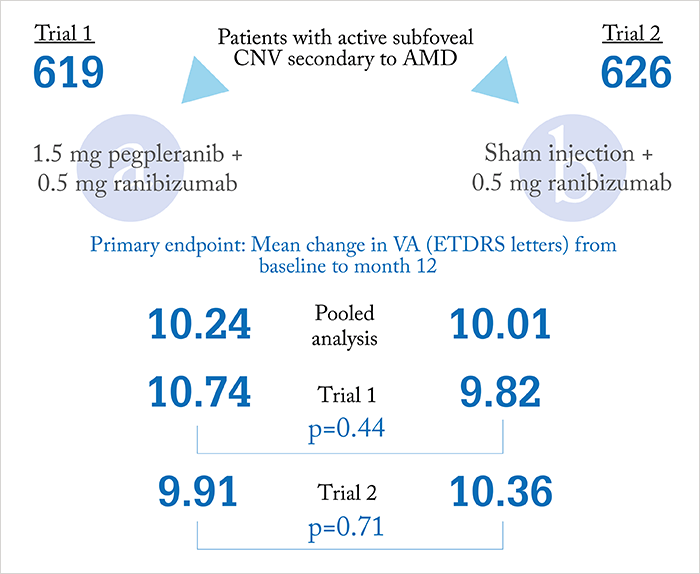
It was looking good – Phase II results of the CAPELLA study (in which pegpleranib and ranibizumab was compared with ranibizumab monotherapy in patients with treatment-naïve neovascular AMD) showed favorable outcomes for the combination (1). But, now, Ophthotech and Novartis have announced that the combination has failed to show superiority over ranibizumab monotherapy in a pair of Phase III trials (NCT01944839 and NCT01940900)(2), (3). The two multisite, randomized controlled trials assessed the anti-PDGF and anti-VEGF combination therapy in over 1,200 patients with neovascular AMD. Here are the topline results from the pooled analysis:
- The pre-specified primary endpoint of mean change in visual acuity at 12 months was not achieved (Figure 1). Patients receiving combination therapy gained a mean of 10.24 ETDRS letters at 12 months compared with 10.01 letters in patients receiving ranibizumab monotherapy.
- At month 12, 24.2 percent of patients receiving combination therapy gained 20 or more ETDRS letters from baseline, compared with 22.1 percent of patients receiving monotherapy.
- More patients in the combination therapy group lost five or more letters at month 12 (12.1 percent vs. 11.2 percent in the monotherapy group).
- In the combination therapy group, 13.5 percent of patients achieved a VA of 20/25 compared with 13.9 percent in the monotherapy group.
- Both treatments were well tolerated at one year. Ocular adverse events reported more frequently in the combination group were related to the injection procedure.
Ophthotech’s CEO, David Guyer comments, “We will continue to analyze the data from these two studies to better understand the trial results.” Vasant Narasimhan, Global Head of Drug Development and Chief Medical Officer at Novartis (who partnered with Ophthotech) adds, “We are confident that underlying data will provide further understanding and guidance on how best to help patients with this disease.” Novartis has other irons in the fire, though: RTH258, a single chain antibody fragment anti-VEGF agent, which is currently undergoing Phase III trial evaluation, with results expected early this year.
References
- GJ Jaffe et al., “Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: a Phase IIb, multicenter, randomized controlled trial”, Ophthalmol (2016). [Article in press]. Ophthotech, “Ophthotech announces results from pivotal Phase 3 trials of Fovista® in wet age-related macular degeneration”, (2016). Available at: http://bit.ly/FovLucPhIII. Accessed December 13, 2016. Novartis, “Novartis provides update on pegpleranib Phase III clinical trial program in patients with neovascular age-related macular degeneration (nAMD or wet AMD)”, (2016). Available at: http://bit.ly/NovartisFovLuc. Accessed December 13, 2016.
