
Michael Pro
Michael Pro, MD, a board-certified ophthalmologist and attending physician specializing in glaucoma at Wills Eye Hospital, Philadelphia USA, adopted the Ahmed ClearPath (ACP) – a novel valveless glaucoma drainage device (GDD) – shortly after its launch in 2019.
“When I saw there was a new valveless tube design available, I was excited to try it,” he says. “And after using it on a few cases, I really began to appreciate its design features, especially the flexibility of the plate and the anteriorly positioned eyelets for fixation.”
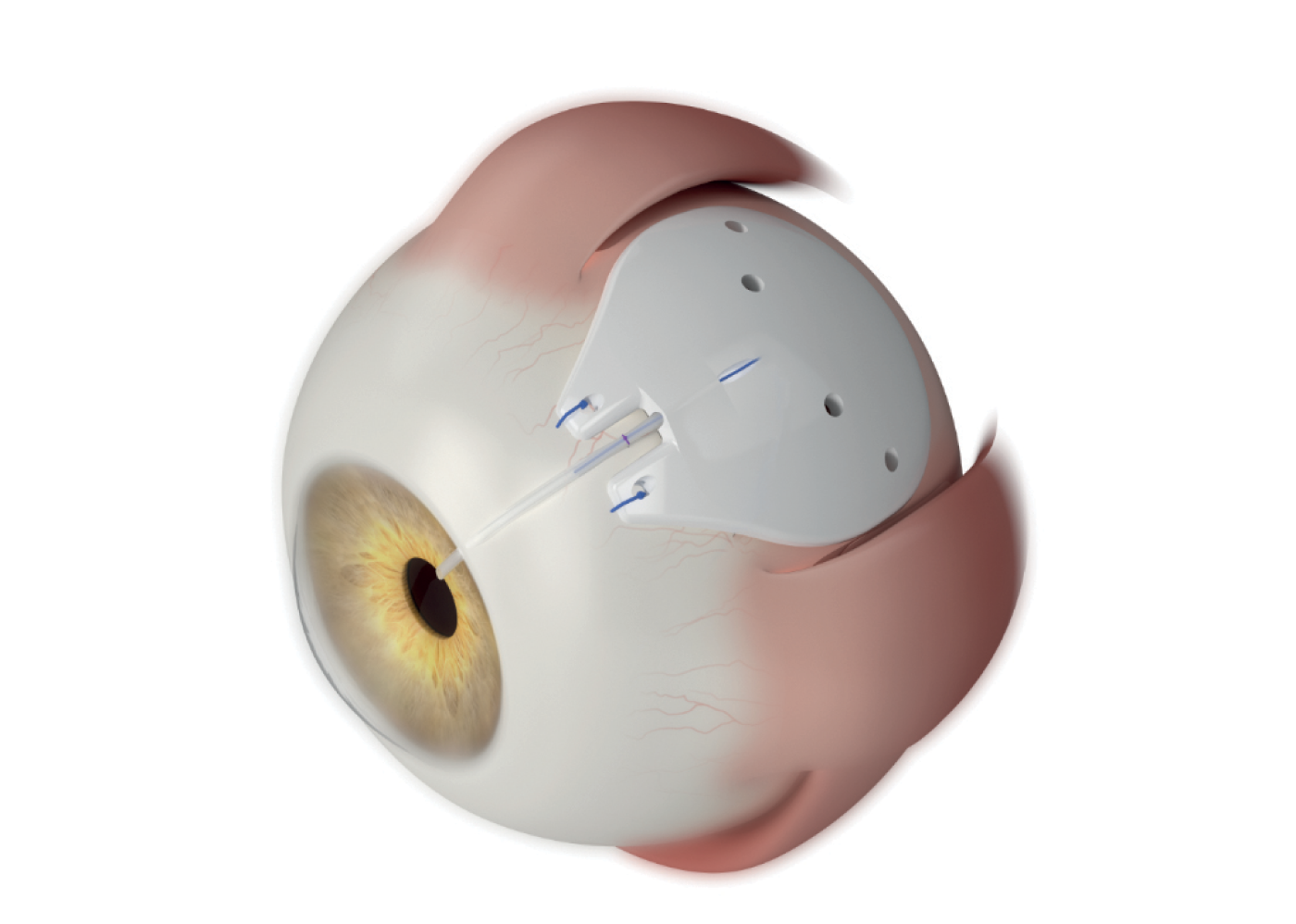
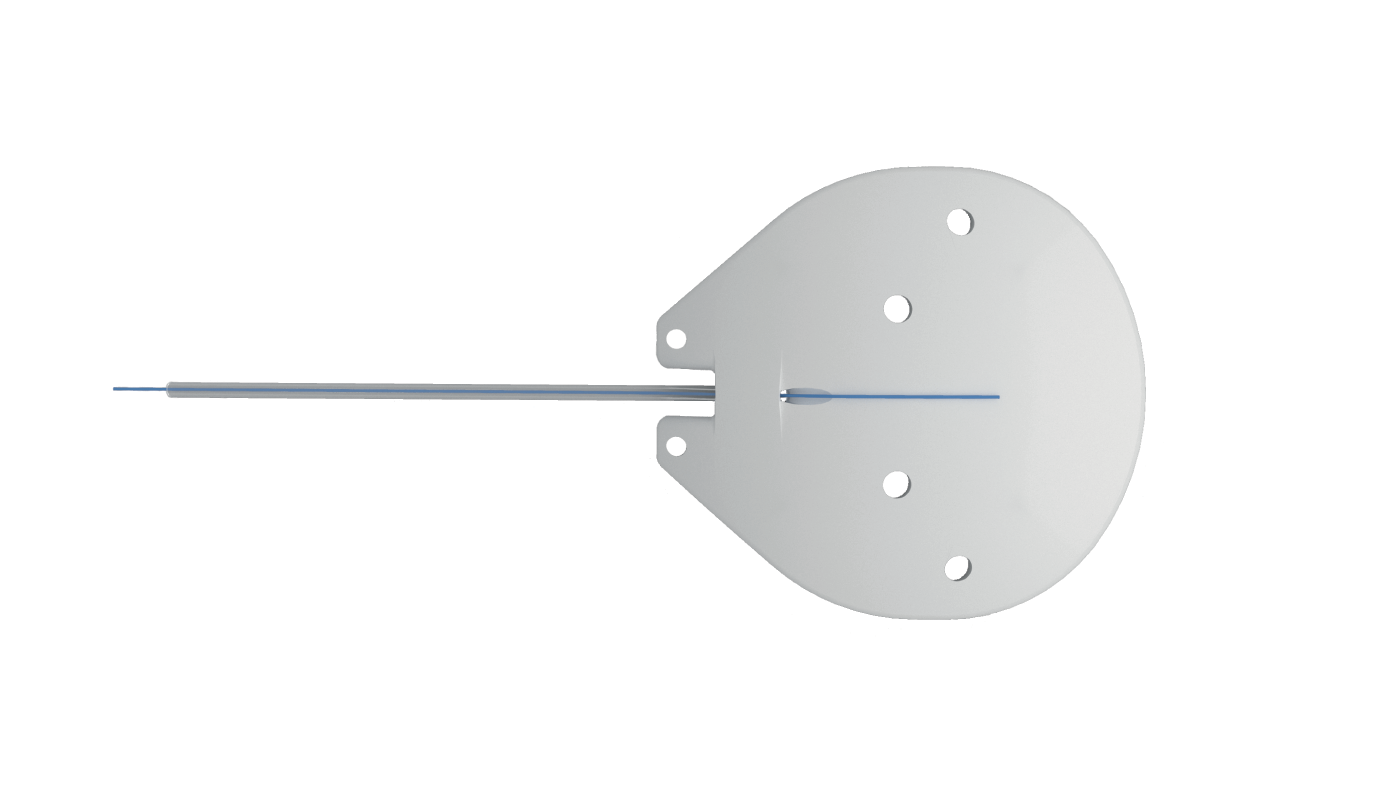
The ACP is available in two sizes – 250 mm2 and 350 mm2. The smaller model facilitates a true single-quadrant implantation between the rectus muscles, eliminating the need for muscle isolation. While the winged design of the 350 avoids attachment points to the rectus muscles, providing stability when positioned beneath the muscles.
“I mostly favor the ACP 250 mm,” explains Pro. “In many cases I’m dealing with more refractory glaucoma, more challenging cases, and patients who have had prior surgery. It allows me more flexibility in terms of where I can put the tube. For example, I can slide it in next to another tube, or slide it more easily over scleral buckles.”
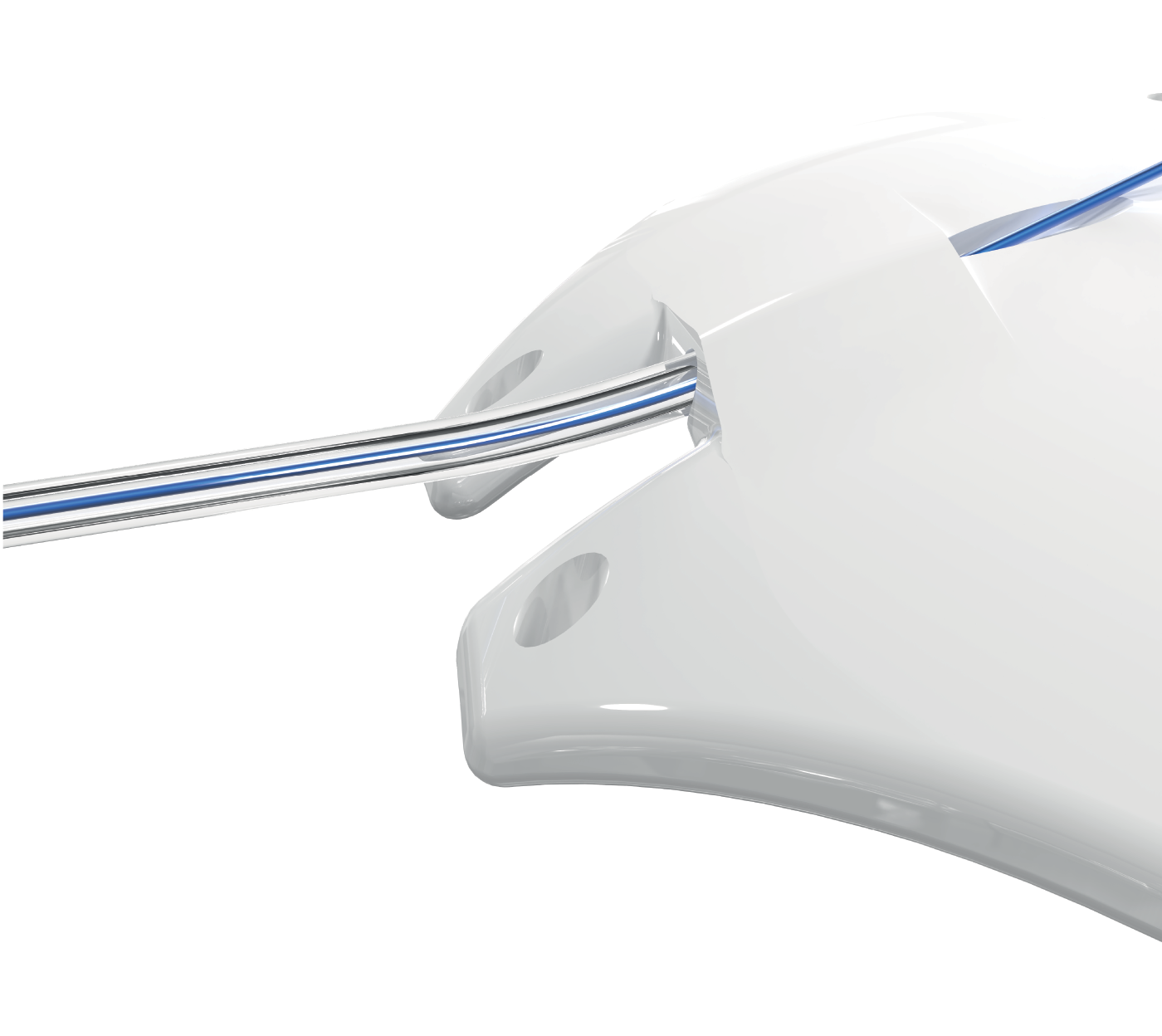
Pro shares his insights on how the ACP 250 has changed his surgical techniques for non-valved implant procedures. He notes: “Previously I would have to do a little more subconjunctival dissection, more conjunctival peritomy, but with the ACP 250, I don't have to engage the muscles so often; I can fold the plate and slide it between the muscles easily. And with the anteriorly fixated eyelets, I’m not as worried about being as far posteriorly for fixating the plate on the eye. I always like to fixate my plates on the globe. And this is another area where the ACP’s plate design offers me surgical flexibility.”
This year, Pro was involved in a research study at Wills Eye Hospital that compared the efficacy and safety of the ACP and the Baerveldt® Glaucoma Implant (BGI) (1). The study looked at 128 eyes of 113 patients (63 ACP, 65 BGI) with similar baseline characteristics and a mean follow-up duration of 19.6 ± 10.8 (median 20.5) months. Both groups achieved significant intraocular pressure (IOP) and medication reduction compared to their baseline. The final intraocular pressure (IOP), best corrected visual acuity (BCVA), and complication rates were similar between the two groups, but the number of medications was significantly lower in the ACP group.

“Wills Eye Hospital is a fairly high-volume tertiary glaucoma practice and referral base, and so we are seeing patients that often have more advanced glaucoma. This study involved patients mostly with primary open angle glaucoma,” Pro explains. “The results, as I expected, were similar to what I’m seeing in my clinical practice. We had very good surgical success – failure rates of less than 10 percent in both the ACP and the BGI groups. What is particularly impressive is how much IOP is being reduced with these devices. But with the ACP group, we also saw slightly less medication at six months and at final follow-up.”
Pro was initially drawn to the ACP due to the limited variety in the U.S. glaucoma implant market, which has been predominantly dominated by BGI. He appreciates having a new option, stating, "With the results I've seen, it makes it a clear choice for my patients." After five years of experience with the device, he confidently confirms that the ACP has become his preferred non-valved glaucoma drainage device for most of his patients.
References
- WS Shalaby et al., “Ahmed ClearPath vs. Baerveldt Glaucoma Implant: A Retrospective Noninferiority Comparative Study,” Ophthalmol Glaucoma. 7, 251 (2024). PMID: 38158079.
