Anti-VEGF drugs have transformed the treatment of a number of retinal diseases: neovascular AMD (nAMD), retinal vein occlusion, diabetic macular edema to name but a few. But their effect doesn’t last forever – most patients will experience some impressive visual gains when therapy commences, but by four years (or sooner), most patients’ VA has declined beyond baseline levels. The likely culprit: pericytes. When new blood vessels grow, the pericytes grow too, coating the vascular endothelial cells, protecting them from anti-VEGF agents, and nourishing them with growth and cell survival factors, including… VEGF. One molecule that recruits pericytes to this task, and promotes their maturation and survival is platelet-derived growth factor (PDGF), and the challenge since this discovery has been to produce antagonists of PDGF receptors.
Pegpleranib (E10030; Fovista, Ophthotech) is one such anti-PDGF agent – a 32-mer pegylated DNA aptamer that selectively binds some of them: PDGF-BB and PDGF-AB homodimers and heterodimers. Preclinical studies have shown that the drug “potently strips neovascular pericytes from the underlying endothelial cells” – which in theory, reopens the endothelial cells to attack by anti-VEGF drugs. However, preclinical data doesn’t always translate into reality: Regeneron recently reported topline results from its Phase II CAPELLA study (1), which compared q4w aflibercept 2 mg monotherapy with q4w aflibercept 2 mg plus the anti-PDGF agent rinucumab (3 mg). The monotherapy was superior to the combination therapy. So the question is: can pegpleranib plus ranibizumab show additional benefit over ranibizumab alone?
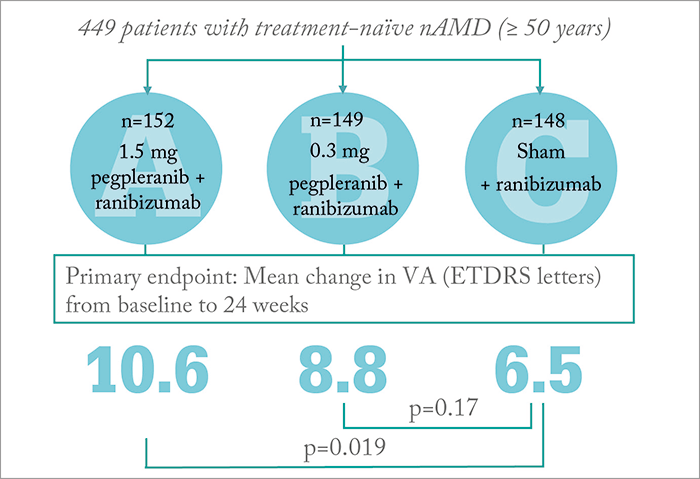
Phase IIb trial results of exactly that comparison in patients with nAMD have now been published, and it looks like the answer is yes (2). In this prospective, multisite, randomized controlled trial that involved 449 patients with treatment-naïve, nAMD, patients were administered monthly regimens of: pegpleranib (1.5 mg [Arm A] or 0.3 mg [Arm B]) plus ranibizumab (0.5 mg), or ranibizumab (0.5 mg) alone (Arm C) for 24 weeks. Here’s what the investigators found (Figure 1):
- Patients in Arm A who received the combination of pegpleranib (1.5 mg) and ranibizumab (0.5 mg) gained a mean of 10.6 letters of vision on the ETDRS standardized chart at 24 weeks, compared with 6.5 letters for patients who received ranibizumab monotherapy (p=0.019).
- A dose-response relationship was observed after four weeks of therapy.
- A greater proportion of patients receiving combination therapy with 1.5 mg pegpleranib had three or more lines of improvement at weeks 12 and 24.
- Combination therapy was well tolerated, with the most frequently reported adverse events being related to the injection itself.
References
- PR Newswire, Regeneron announces Phase2 study of aflibercept co-formulated withrinucumab (anti-PDGFR-beta) shows nobenefit over aflibercept alone in neovascular age-related macular degeneration Available at: bit.ly/rincumab. Accessed November 1, 2016. GJ Jaffe et al., “Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase IIb, multicenter, randomized controlled trial”, Ophthalmology (2016). [Article in press].
