The burden of dry eye disease (DED) is easy to underestimate. It has many causes, is thought to affect up to 100 million people globally, but is both underdiagnosed and undertreated. The reason: DED has no standard natural history, and a highly variable symptom profile at different stages of the disease. Further, there’s often a discordance between signs and symptoms (1,2): a patient can have severe symptoms, yet show no sign of ocular surface damage, whereas others have advanced ocular surface damage, yet report no symptoms. In order to address this possible lack of correlation between patients’ subjective irritative ocular symptoms (as determined by, for example, questionnaires) and the results of commonly performed objective tests, such as corneal fluorescein staining (CFS) and tear film break-up time (TFBUT) it is important to perform a thorough diagnosis.
A better diagnosis
Thankfully though, improved diagnostic algorithms and questionnaires are helping to identify more of those patients with the severe forms of the disease. The ODISSEY European Consensus Group recently reviewed the clinical and scientific challenges in the diagnosis and management of severe DED, and generated an algorithm that identifies the criteria most relevant to the patient (3), producing a two-step scoring algorithm for diagnosing severe DED. Just two criteria – a symptomatic assessment with the Ocular Surface Disease Index (OSDI) questionnaire and an evaluation of ocular surface damage by CFS – were deemed sufficient as a frontline assessment of DED severity for the majority of patients. Where there was discordance between signs and symptoms, further assessments were recommended with eight “determinant” factors being listed – including biomarkers of inflammation and apoptosis as well as osmolarity, meibomian gland disease and eyelid inflammation. Their presence, in addition to OSDI score ≥33 or CFS score ≥3 on the Oxford scale, was accepted as diagnosis of severe DED. However, it’s long been known that most patients find long questionnaires boring, so it’s important to keep the number of questions down to a minimum: the OSDI questionnaire asks only twelve. However, OSDI only addresses symptom frequency but not intensity. DED – particularly severe DED – negatively impacts patients’ quality of life (QoL), which is in turn, poorly associated with clinical findings (4–7). In a bid to address shortcomings of OSDI, a new questionnaire, developed and validated first in Japan (and now in Germany, France and the UK) – Dry Eye-Related Quality-of-Life Score (DEQS) – successfully combines frequency, intensity, as well as QoL in just 15 questions, and was found to have good reliability, validity, specificity, and responsiveness for all three factors (8). It may, therefore, provide a rapid and easy first assessment of DED severity.Improving outcomes by combating inflammation
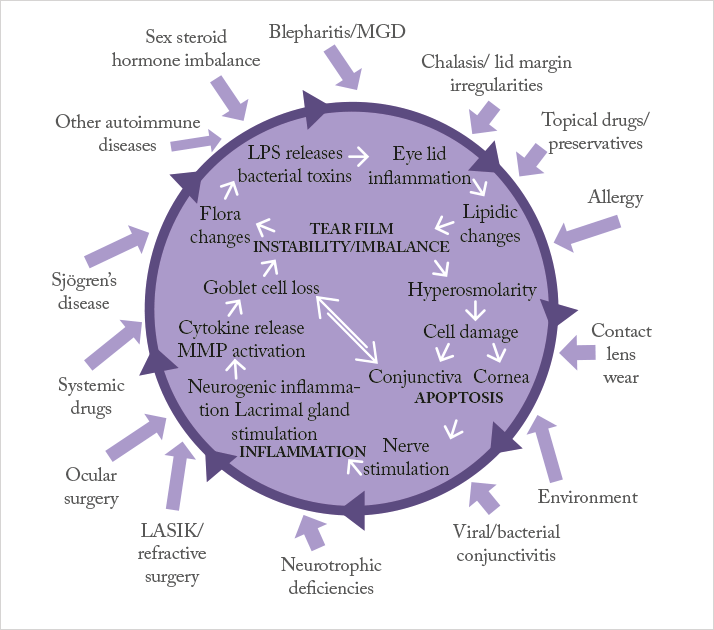
Treating patients with mild-to-moderate DED is relatively simple: artificial tears and lubricants can suffice. Oral and topical tetracyclines are sometimes employed in patients with certain forms of dry eye that result from ocular rosacea, or chronic posterior blepharitis/ meibomianitis, where it helps ease meibomian gland dysfunction (9). But treating severe DED requires a different approach. If we refer to Figure 1 – the vicious circle theory of dry eye – artificial tears and lubricants can help address the dry eye symptoms caused by the factors on the outside of the vicious circle, but they do nothing to address the disease processes at its core. Anti-inflammatory agents can get inside the vicious circle and disrupt it, resulting in a therapy that addresses the causes of DED, not just the symptoms (10). Ultimately, topical anti-inflammatory treatments options able to be used in this condition are few and come down to corticosteroids and ciclosporin (9).Corticosteroids have a rapid and powerful anti-inflammatory effect, and have been shown to improve the signs and symptoms of DED (9). However, this can come at a cost: their ocular side- effect profile, with raised intraocular pressure being a possible short-term risk (11), and cataract development in phakic patients being a longer-term possibility (12). Ciclosporin, on the other hand, controls inflammation by a completely different mechanism of action; it avoids the potentially deal-breaking side- effects of corticosteroid therapy, yet still provides anti-inflammatory efficacy. Although ciclosporin takes longer to produce an anti-inflammatory effect than corticosteroids, its use carries fewer risks and should be safer for chronic application. The only problem is that, up until recently in Europe, there was no EU-approved, commercially available ciclosporin-containing eyedrop formulation available.
Meeting that unmet need
That was yesterday’s challenge. Ikervis, (ciclosporin 1 mg/ml eye drops, emulsion, Santen Oy, Finland), has recently received marketing approval in the EU (13) for the “treatment of severe keratitis in adult patients with dry eye disease, which has not improved despite treatment with tear substitutes.” The posology is simple: a single drop into each affected eye once daily at bedtime. The EU-wide approval in May 2015 was based on the results of a multicenter, randomized, controlled, double-masked clinical program, which enrolled 738 patients and compared Ikervis with an active control vehicle (13–15). In general, Ikervis is well-tolerated – even with long-term treatment of up to 12 months, but there are two things patients should be aware of. First, the active ingredient of Ikervis, ciclosporin, gives rise to the therapy’s most common side-effect: eye pain or irritation – often described by patients as a burning sensation upon instillation – but which is normally mild-to-moderate in intensity (13). Second, it will take some time before ocular surface disease starts to improve – Ikervis’ effects aren’t immediate, but over the longer term, it’s effective in reducing ocular surface damage and inflammation, and may prevent DED from worsening (15).Smaller droplet size makes for a smarter formulation
Ikervis’ formulation is worth a closer look. Ikervis is a cationic oil-in-water emulsion of 1 mg/ml ciclosporin, based on Santen’s Novasorb technology (16). The positively charged nano-sized droplets of the emulsion electrostatically adhere to the negatively charged mucins on the ocular surface, thereby improving ocular retention and absorption (Figure 2), and the lipids in the formulation support the stabilization of the tear film, too (16). The fact that the droplets are nano-sized is important: as droplet size reduces, the surface area to volume ratio increases, meaning a greater total surface area of the emulsion is exposed to the ocular surface – in essence, the eye “sees” more of the eyedrop, which is why the innovative cationic formulation of Ikervis makes once-a-day dosing possible (13,17).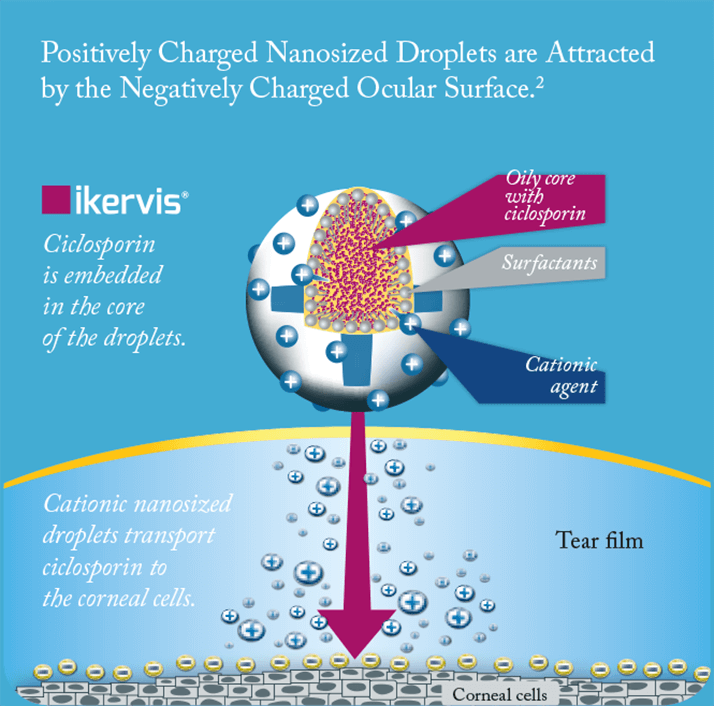
Ultimately, the introduction of Ikervis into the European market means three things. First, unlike artificial tears and lubricants, Ikervis addresses the underlying inflammatory processes present in severe DED. Second, its formulation requires no refrigeration (since it can be stored at room temperature) and has a three- year shelf-life (13). And finally, it is the only approved ciclosporin eyedrop available commercially in the EU today – bringing a novel treatment option to patients with severe keratitis in DED who, until now, had no access to this class of therapy.
References
- KK Nichols, et al., Cornea, 23, 762–770 (2004). PMID: 15502475.
- BD Sullivan, et al., Acta Ophthalmol, 92, 161–166 (2014). PMID: 23279964.
- C. Baudouin, et al., Br J Ophthalmol, 98, 1168–1176 (2014). PMID: 24627252.
- S Vitale, et al., Health Qual Life Outcomes, 2, 44 (2004). PMID: 15341657.
- M Li, et al., Invest Ophthalmol Vis Sci, 53, 5722–5727 (2012). PMID: 22836767.
- M Li, et al., Curr Eye Res, 36, 1–7 (2011). PMID: 21174591.
- KW Kim, et al., Invest Ophthalmol Vis Sci, 52, 7954–7958 (2011). PMID: 21896858.
- Y Sakane, et al., JAMA Ophthalmol, 131, 1331–1338 (2013). PMID: 23949096.
- [No authors listed], Ocul Surf, 5, 163–178 (2007). PMID: 17508120.
- C Baudouin, J Fr Ophtalmol, 30, 239–46 (2007).
- R Jones, DJ Rhee, Curr Opin Ophthalmol, 17, 163–167 (2006). PMID: 16552251.
- L Bielory, Immunol Allergy Clin N Am, 28, 189–224 (2008). PMID:18282552
- IKERVIS 1 mg/mL eye drops, emulsion. Summary of product characteristics. Available at: bit.ly/ikervis. Accessed July 15, 2015.
- European Medicines Agency. IKERVIS Public Assessment Report. EMA CHMP/473489/2014. Available at: bit.ly/ ikervisEPAR. Accessed July 15, 2015.
- European Medicines Agency, EPAR summary for the public, EMA/56994/2015. Available at: bit.ly/ikervisEPARsummary. Accessed July 15, 2015.
- F Lallemand, et al., J Drug Delivery, 2012:604204 (2012).
- P Daull, et al., Cornea, 32, 345–54 (2013). PMID: 23023401.
Indication: Treatment of severe keratitis in adult patients with dry eye disease, which has not improved despite treatment with tear substitutes.
Dosage and administration: IKERVIS® treatment must be initiated by an ophthalmologist or a healthcare professional qualified in ophthalmology. The recommended dose is one drop of IKERVIS® once daily to be applied to the affected eye(s) at bedtime. Response to treatment should be reassessed at least every 6 months. To reduce systemic absorption, advise patients to use nasolacrimal occlusion and to close the eyelids for 2 minutes after instillation. If more than one topical ophthalmic product is used, 15 minutes should separate their administration. IKERVIS should be administered last.
Contraindications: Hypersensitivity to any of the ingredients. Active or suspected ocular or peri-ocular infection.
Warnings and Precautions: Use with caution in patients with a history of ocular herpes. Contact lenses: Patients wearing contact lenses have not been studied. Monitor carefully inpatients with severe keratitis. Contact lenses should be removed before instillation of the eye drops at bedtime and may be reinserted at wake-up time. Concomitant therapy: Use with caution in patients with glaucoma, especially in those receiving concomitant beta-blockers which are known to decrease tear secretion. Immune system effects: Medicinal products which affect the immune system, including ciclosporin, may affect host defences against infections and malignancies. Contains cetalkonium chloride which may cause eye irritation.
Interactions with other medicinal products: Coadministration with eye-drops containing corticosteroids may potentiate effects on the immune system.
Pregnancy and Breast Feeding: Not recommended in women of childbearing potential not using effective contraception or during pregnancy unless the potential benefit to the mother outweighs the potential risk to the foetus. Benefits of treatment must be weighed against the benefits of breast feeding.
Driving and using machines: Moderate influence on the ability to drive and use machines. If blurred vision occurs on instillation, the patient should be advised to not drive or use machines until their vision has cleared.
Undesirable Effects: Consult SmPC for full details. The most common adverse reactions in clinical studies were eye pain, eye irritation, lacrimation, ocular hyperaemia and eyelid erythema. Patients receiving immunosuppressive therapies including ciclosporin, are at an increased risk of infections.
Special Precautions for Storage: Do not freeze. After opening of the aluminium pouches, the single-dose containers should be kept in the pouches in order to protect from light and avoid evaporation. Discard any opened individual single-dose container with any remaining emulsion immediately after use.
Package quantities and basic NHS cost: 30 x 0.3ml single-dose containers £72.00.
Product Licence Holder: Santen Oy, Niittyhaankatu 20, 33720 Tampere, Finland (PL 16058/0012) (EU/1/15/990/001 & 002)
Date of Authorisation: March 2015 Legal Category: POM
Date of last revision of Prescribing Information: 07/07/2015
IKERVIS® is a registered trademark of Santen Pharmaceuticals Co., Ltd. Job code: STN 0617 IKV 00004a. Adverse events should be reported.
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Santen UK Limited
(Email medinfo@santen.uk or telephone: 0845 075 4863). 1. SANSIKA study, Santen Data on File 0002 Job code: STN 0817 IKV 00021(eu). Date of preparation: August 2015.
