
Entirely self-retaining, LacriJet® (FCI S.A.S., Paris, France) is a revolutionary injection system that has been specifically designed to reduce operating time – both in the intubation phase and removal. The world’s first injectable monocanalicular intubation stent indicated for congenital or acquired nasolacrimal duct obstruction (NLDO) and canalicular laceration, LacriJet® consists of a single-use injector handpiece into which a silicone tube is preloaded inside a metallic guide.
The device design – an FCI-exclusive – is unique in both its form and functionality. But behind the smart design lies simple operation. First, the LacriJet® is introduced into the nasolacrimal duct; once in position, the sliding piston is retracted and the silicone intubation is released. The intubation is maintained in place at the punctum by a plug-like fixation head: no nasal retrieval, no knots, and no suture necessary. The device is available in seven lengths: five lengths for nasolacrimal stenosis – from 30 mm to 50 mm – to restore the patency of the lacrimal drainage system both in children and adults; and two shorter lengths for canalicular laceration – 15 mm and 20 mm – to prevent future canalicular obstruction due to scar tissue formation.
To understand why the LacriJet® is revolutionary, it is important to understand the condition it treats. NLDO, a blockage of the lacrimal drainage system, can be either inherited or acquired following trauma, viral conjunctivitis, acute dacryocystitis, or the use of topical antiviral medications. Since 1984, all FCI lacrimal intubations (monocanalicular and bicanalicular) have been designed to treat a specific obstruction: first, according to its location in the tear drainage system and, secondly, according to pathology severity.
These multiple options enable surgeons to select the most appropriate device to treat their patients, in terms of techniques (“pulled” or “pushed”), preference, and usability. As a “pushed” technique, the LacriJet® is significantly less traumatic than “pulled” on the nasal mucosa as there is no need for retrieval. It is also more user-friendly for the patient, as the lack of knots or sutures means there is little-to-no blood loss during or after the procedure.
The device is equally effective when it comes to canalicular repair. Back in 1989, FCI invented the Mini-Monoka® – known as the gold standard for canalicular repair. This short silicone monocanalicular stent was specifically designed for repairs involving the external two-thirds of the canaliculus to avoid future blockage caused by scar tissue. However, the tube placement procedure was considered by some to be too complicated for some inexperienced doctors, as the silicone tube was soft and needed to be passed through the lacerations for suturing.
The LacriJet® 15 mm and 20 mm have been specifically designed to facilitate the procedure, using a metallic probe to act as a catheterization guide between the distal and proximal portions of the canaliculus. The silicone tube is then released and left in place to avoid future scar tissue-induced blockages. Simple, yet effective. The LacriJet®’s innovative design can be attributed to Bruno Fayet, MD, renowned oculoplastic surgeon, lacrimal field specialist, and long-time FCI collaborator. Fayet also contributed to the design of many existing monocanalicular lacrimal intubations from FCI, including the Mini-Monoka®, Self-threading Monoka® (Ritleng type), Monoka® of Fayet (Crawford guide), Monoka®, and Masterka®.
The products mentioned may not be registered in your country. Please contact your local distributor to find out what is available in your area.
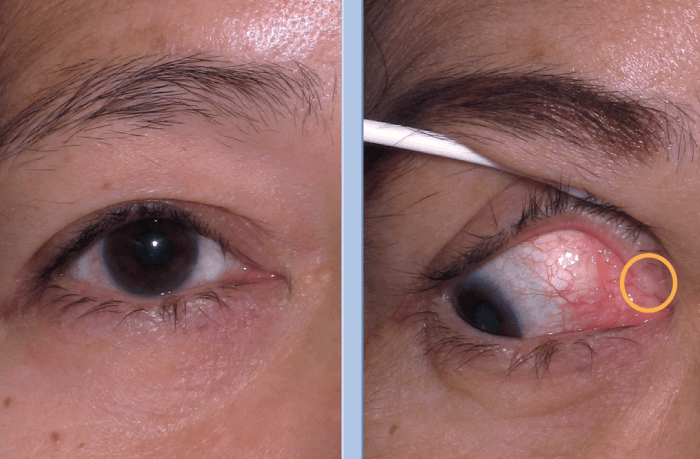
Cases of nasolacrimal duct obstruction, epiphora and canalicular lacerations are usually referred to me by my colleagues and trainees. The most challenging epiphora cases are those with nasolacrimal duct obstructions from congenital bony anomalies, and with canalicular obstruction or stenosis with long distance occlusion (involving the proximal, middle, distal and common canalicular obstructions).
I have found FCI’s LacriJet® very useful for patients with focal stenosis of the upper or lower canaliculus. I also use it for patients with focal or partial NLDO after doing a recanalization procedure, and for those suffering from a recurrence or reobstruction after a transcanalicular endoscopic lacrimal duct recanalization. The device is user-friendly, and it saves precious procedure time.
The LacriJet® is preloaded into an injector, which protects the silicone stent during insertion or injection into the tear ducts. It has a selfretaining punctal fixation, removing the need to secure the end of the tube in place in the inferior meatus with knots or sutures. Prior to inserting the LacriJet®, the punctum should be dilated, to allow for the introducer’s external diameter of 0.9 mm. I have found the monocanalicular stent to carry less risk of injury to the intact canaliculus than a bicanalicular silicone stent.
Javate is Past President of the Philippine Society of Ophthalmic Plastic and Reconstructive Surgery; Founding President of the Asia-Pacific Society of Ophthalmic Plastic and Reconstructive Surgery; President of 10th International Society of Dacryology and Dry Eye; and Fellow of the American Society of Ophthalmic Plastic and Reconstructive Surgery.
