Imaging modalities like OCT, and AO-SLO have transformed our ability to stage and diagnose retinal disease. But in many respects it’s only showing you the pathologic structural changes that occur after the damage has been done. Take the example of diabetic retinopathy. By the time a patient begins to notice problems with their vision, they’ve lost a significant portion of it – and a hefty number of cells in the retina too. Much like age-related macular degeneration (AMD) and glaucoma, interventions at this point are almost palliative: current therapies try to slow the diseases progression, but they can’t replace what’s lost. But what if you could detect disease processes before permanent damage has happened? At a point where early, disease-altering interventions – some as simple as lifestyle changes – could be made? Sound unrealistic? Apparently not. It seems there is a way - by using mitochondrial function as a marker of retinal health.
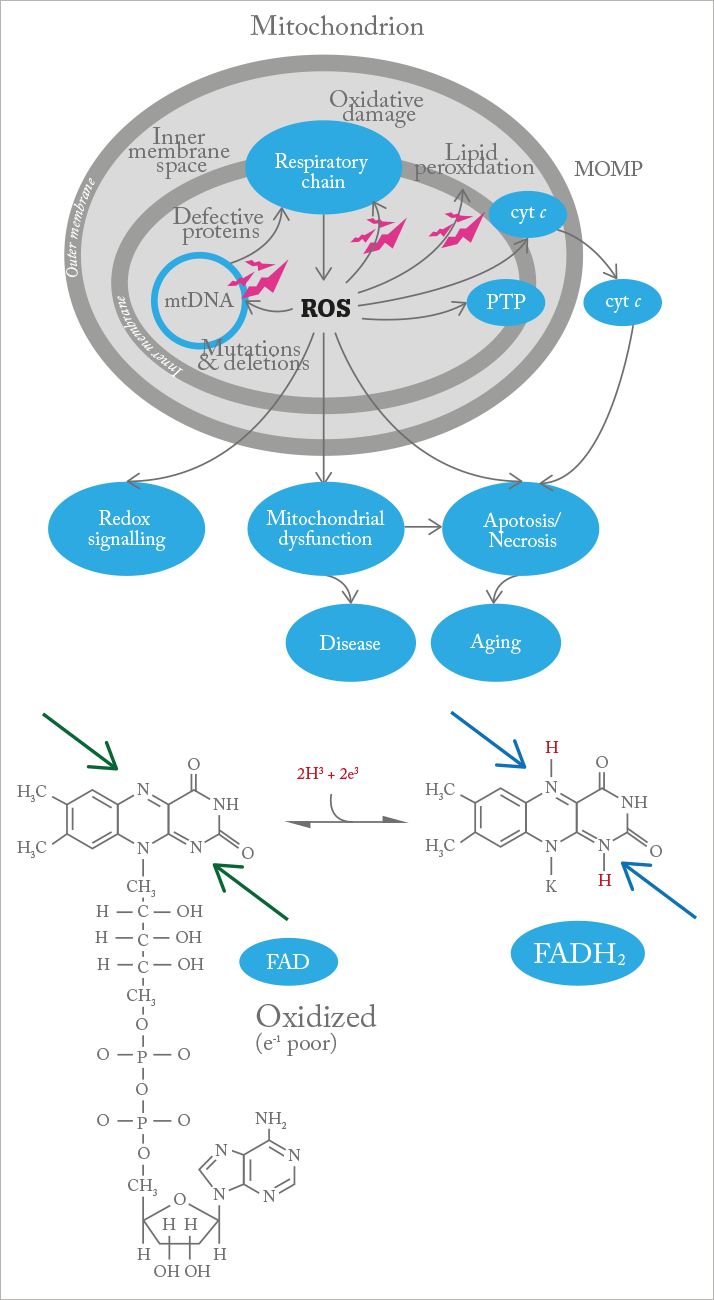
As you’ll no doubt remember from high school, mitochondria are the organelles found in all eukaryotic cells that perform aerobic respiration to produce chemical energy in the form of adenosine triphosphate (ATP). They’re also involved in cell signaling, differentiation and apoptosis. And there’s a growing body of evidence that mitochondrial dysfunction can have big implications for retinal disease (1). In the axons and somata of retinal ganglion cells, the oxidative phosphorylation machinery within the mitochondria are impaired by reactive oxygen species (ROS), but the organelles themselves are suspected to be a major ROS source within the eye. This can result in a vicious cycle of oxidative stress accumulating over time, eventually leading to retinal ganglion cell (RGC) death, which is likely to be important in glaucoma and optic neuropathies. Oxidative stress is also a major factor affecting other retinal cells depending on the disease – for example, photoreceptors and retinal pigment epithelium in AMD. This metabolic deterioration is thought to play a part in diabetic retinopathy, glaucoma, AMD, and other retinal diseases (2), and mutations in mitochondrial DNA - both inherited and acquired - are implicated in a range of conditions, including Leber hereditary optic neuropathy (LHON) (3) and chronic progressive external ophthalmolplegia (4).
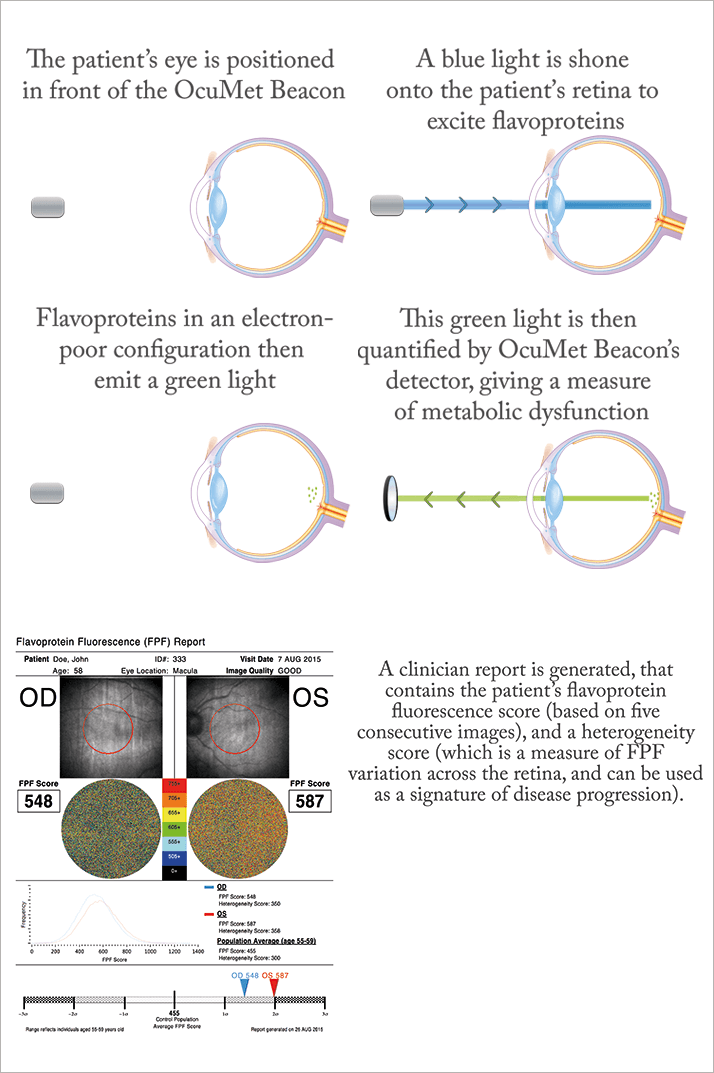
Now, a device has been developed that provides noninvasive mitochondrial imaging, giving insight into the metabolic function of retinal cells and flagging problems at a point before cell damage occurs. When the mitochondria malfunction, flavoproteins, which act as electron acceptors in the electron transport chain, are left in an electron-poor (oxidized) state (see Figure 1). By exposing the retina to blue light, the OcuMet Beacon can detect the green fluorescence they emit, which can then be quantified as an FPF (flavoprotein fluorescence) score (see “How Retinal Metabolic Analysis Works”).
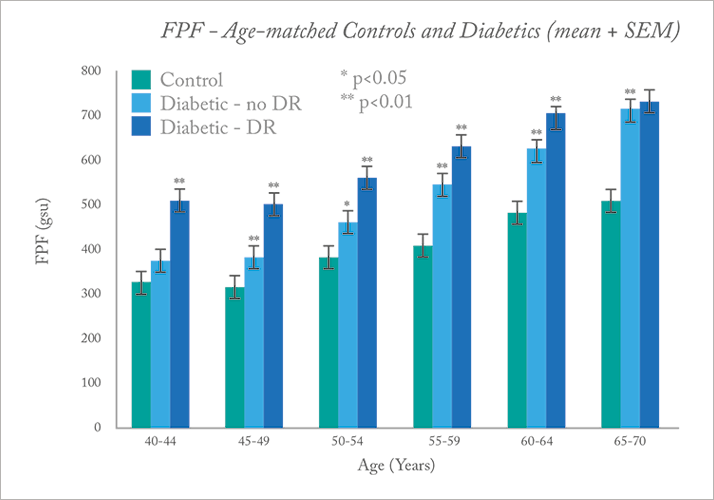
The technique is still being validated, but initial studies have shown potential. For example, in diabetic patients versus age-matched controls, FPF levels were found to be significantly higher in patients with diabetes, including those with no visible retinopathy, implying that retinal metabolic stress is apparent before anatomical changes occur (see Figure 2) (5). In a small study of primary open angle glaucoma, again, FPF levels were found to be higher in glaucoma patients compared with controls, with greater variability between fellow eyes (6).
For glaucoma, the technique could offer a promising additional method for staging the disease, as IOP and optic nerve imaging can be fairly crude assessments – IOP doesn’t always correlate well with the extent of vision loss and once the optic nerve has become damaged, the disease has already progressed quite a bit. But the compelling uses are clearly screening – catch a disease and effect a sight-saving intervention before cellular damage occurs – and endpoint assessment in clinical and preclinical trials of therapeutic intervention. What’s holding the Beacon back today is data. The correlation between FPF and subsequent damage to the retina or the optic nerve needs to be more completely characterized – and that’s something that’s only built over time. But normative databases exist already, and are continually being expanded, with every new datapoint contributing to a better understanding of the relationship between FPF, oxidative stress, normal aging, and disease status. However, it doesn’t take precognitive abilities to see that in a decade’s time, this technology might transform how patients are screened – and quite possibly the interventions too.
Richard Rosen, Professor of Ophthalmology at New York Eye and Ear Infirmary of Mount Sinai shares his experiences using the OcuMet Beacon for retinal metabolic analysis.
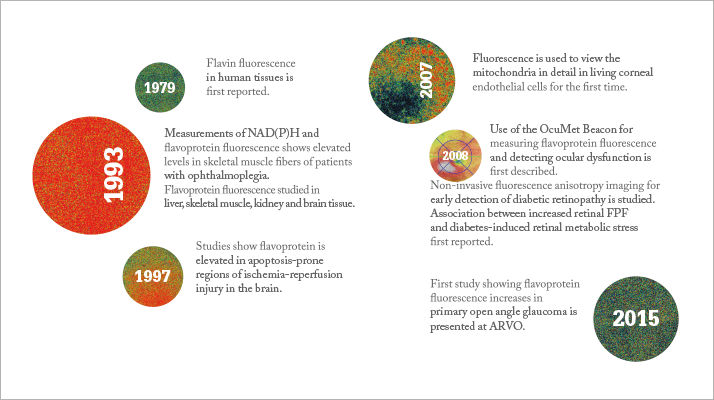
How did you get involved in the development of the Beacon? I have been following its development for some time. I have a lot of experience studying prototype imaging devices and trying to identify useful parameters – for example I collaborated with Adrian Podoleanu and his team when developing the simultaneous OCT/SLO/ICG imaging system. At the same time, I’m primarily a clinician, so my main interest is getting these technologies into the clinic, to find out how I can use them to treat or monitor my patients. I had some mutual friends in the team that was working on the OcuMet Beacon, and I told them I was really interested. I’m now helping to gather data, but I’m not employed by them on a research or collaborative basis. Why was it previously difficult to study? The biggest problem was that many of the processes occurring in the mitochondria occur at fluorescence wavelengths that aren’t readily accessible. Two-photon imaging has been explored, but as it currently stands, it’s still far too phototoxic for clinical use. There was also the work done by Ralph Zuckerman, who used fluorescence anisotropy to look at changes in mitochondrial walls, but this never reached the clinic. With this device, you’re looking at a very narrow bandwidth, so it’s very targeted, and less harmful to the eye.
What have been your findings so far? Flavoprotein fluorescence seems to give us a good indication of oxidative stress levels in the mitochondria, providing the potential for monitoring changes to cell metabolism that later result in structural changes to the eye. An indication that a drug or intervention is working, or improving overall tissue metabolism, before structural changes become apparent, would be fantastic. I think this truly could be the next big leap in diagnosing, monitoring and treating retinal disease. As we are all aware, there are many spectacular new imaging devices out there, from the latest OCT instruments to adaptive optics. We’ve explored these extensively in our institute, and they’re very helpful, but by and large, they simply can’t give us this kind of early functional data. We need to explore this area further, in order to understand how these elevated levels of oxidative stress can be related to retinal health. The technology is still in the relatively early stages, but we’re finding that there’s a huge interest in being able to monitor mitochondrial function, as it’s becoming obvious from a lot of research that this dysfunction is likely to be central to many diseases.
What retinal diseases have you been focusing on? We’re both looking at normative changes, and some specific diseases, namely Leber hereditary optic neuropathy (LHON), diabetic macular edema (DME) and glaucoma. LHON – a condition caused by mitochondrial DNA mutations – is one we’re very interested in. We have a cohort of patients we’re following in our neuroophthalmology group. Currently, there is no treatment for the disease. A number of pharmaceutical agents have been trialed, to try and bolster the electron transport mechanism in the mitochondria and thereby overcome the effects of the mutation, but these haven’t met with much success. However, there are a number of new agents currently being studied – for example, the group Stealth BioTherapeutics is doing some promising work in this area – and the effects of these therapies could be studied using retinal metabolic analysis. In patients with diabetic eye disease, we want to observe the rate of change in oxidative stress, in terms of response to treatment. We’ve observed that patients treated with anti-VEGF agents for DME have also shown reduction in FPF levels. This suggests a possible re-normalization or improvement in function. But the correlation isn’t perfect – it’s both challenging and incredibly interesting! Our next project in this area is to examine the effects of subthreshold laser treatments used to stimulate heat shock protein response – these are incredibly subtle treatments, which are not easily monitored, as they don’t really cause structural changes. Another exciting area is glaucoma. In a way, glaucoma is a particularly difficult disease, as current monitoring methods can only detect it very late in the process. You can look at the intraocular pressure (IOP) and watch to see how quickly the nerve disappears at a certain pressure, but we don’t have any earlier indications. Interestingly, in one of our patients with high IOP, when we looked at mitochondrial dysfunction we found very high levels of FPF. When IOP was lowered to a more normal pressure, FPF levels also came down, close to what they would be for an age-matched control. Although we don’t have a great normative database for glaucoma yet, we presented some of our preliminary research at ARVO 2015 (6). We have definitely showed that FPF levels are higher in glaucoma patients than in age-matched controls. This information could potentially be used to ensure that patients are receiving optimal treatment. One of the issues glaucoma specialists face is that, although they may have a target pressure, it’s based on the nomogram of a large number of patients, and may not give the right target for individual patients. It’s hoped that in the future you could use retinal metabolic analysis as a means of monitoring – and minimizing – oxidative stress.
What are the challenges? We need to distinguish age-related changes from the impact of a disease process, or the effect of a treatment or drug. Like any technology, it requires a lot of engineering and clinical experience to validate these things over time, because you’re dealing with very small signals, and there are a lot of sources of noise. The physicist Ran Ziemer, who was involved in the development of a device called the retinal thickness analyzer, once said to me “the thing with any new technology is that you’re already caught in a bind – when it comes to what parameters you want to use, you only understand what you know already.” And it’s true – the challenge with measuring a new parameter is figuring out where it fits in with the knowledge you already have, and what it adds.
How far is this technology from the clinic? Well, right now we’re working with a laboratory model. As a reference database is built up, it’s going to be important in determining whether it will be possible to create a clinically viable device that isn’t prohibitively expensive. Right now, although the prototype is very promising, it’s detecting a very, very small signal, and requires a sensitive cooled EMCCD camera, which is not a cheap tool. Achieving this level of sensitivity in consumer electronics will take some work – but from what I’ve seen, I think the next generation will be a lot less costly. The device will also need approval from regulatory agencies like the FDA, and we’ll need to wait and see what they’ll find acceptable in terms of reproducibility and consistency. I don’t have too much information on that side of things, but I do see good consistency in patients I’ve looked at, and I would guess that as more data is acquired, things will improve. It’s probably still a few years away, but I believe it’s getting there.
How can it help with disease management? We’re very interested to see if we can spot earlier effects of drugs, rather than waiting for a structural change. That would be tremendous. I’m fortunate as a retina specialist that, if I treat patients with an anti-VEGF drug, I’ll generally get a quick and visible response. In glaucoma, you don’t get much of a structural response after you’ve normalized IOP. Years ago it was shown that cupping actually improved in patients if you lowered pressure within a certain period of time. But by and large, this disease is relatively silent. If you had an additional index to monitor, you may be able to pick up very early changes, both during the disease process, and to see the effects of the pharmaceutical interventions you’re using. This type of monitoring could also offer a unique way to assess the effects of other interventions such as gene and stem cell therapies.
What about screening? The potential is there. With many of the current imaging methods, as your demand for resolution increases, there are less patients you can screen – patients with significant cataract, or small pupils, or perhaps dry eye, become unsuitable candidates. We’ve been studying microvascular changes in diabetics with our adaptive optics system for years, and although you can see a lot, it’s not an ideal screening tool. Something like this is easily tolerated by most patients, it’s very fast, and it could give you an early signal that something’s wrong – by the time you’re observing anatomical changes, the problem has already been present for some time. In particular, it could have a lot of utility in identifying “prediabetic” patients – we know there are patients who report that their vision is affected very early on, due to subtle lens changes. In patients with consistently high sugar levels, this will affect their levels of oxidative stress, and make it possible for the system to pick them up. So if we had a relatively inexpensive version of this device, it could be coming to the ophthalmologist’s office soon – but the goal would really be to get it into the hands of the hospital doctors, to identify disease as early as possible. We have so many great imaging tools in ophthalmology, but I think, once it’s been developed and validated further, something like this could really bring ophthalmology to the masses, in terms of screening, and catching disease as early as possible.
References
- M Barot, et al., “Mitochondrial dysfunction in retinal diseases”, Curr Eye Res, 36, 1069–1077 (2011). PMID: 21978133. GS Gorman, RW Taylor, “Mitochondrial DNA abnormalities in ophthalmological disease”, Saudi J Ophthalmol, 25, 395–404 (2011). PMID: 23960954. C Meyerson, et al., “Leber hereditary optic neuropathy: current perspectives” Clin Ophthalmol, 26, 1165–1176 (2015). PMID: 26170609. SG Elner, et al., “Retinal flavoprotein autofluorescence as a measure of retinal health”, Trans Am Ophthalmol Soc, 106, 215–222 (2008). PMID: 19277237. MG Field, at al., “Rapid, noninvasive detection of diabetes-induced retinal metabolic stress”, Arch Ophthalmol, 126, 934–938 (2008). PMID: 18625939. A Pinhas et al., “Macular mitochondrial flavoprotein autofluorescence in eyes with primary open angle glaucoma”, IOVS, 56, ARVO E-abstract 3983 (2015).
