Aflibercept is currently the new kid on the block when it comes to marketed anti-VEGF agents. Like ranibizumab, bevacizumab (and pegaptanib) before it, it has a number of potential therapeutic uses in age-related ocular disease: wet age-related macular degeneration (AMD), myopic choroidal neovascularization, retinal vein occlusion, and diabetic macular edema (DME). The questions is: which one is best for which disease?
Establishing that is easier said than done. Understandably, given the costs involved, there’s a fair amount of reticence among pharmaceutical companies to run head to head trials of their drugs versus the competition. This is where the US government has stepped in. As with the CATT trial (which compared ranibizumab with bevacizumab in patients with neovascular AMD, [1]), the National Eye Institute (NEI) has funded the DRCR.net’s Protocol T trial, which compared aflibercept, bevacizumab and ranibizumab for the treatment of DME (2).
Establishing that is easier said than done. Understandably, given the costs involved, there’s a fair amount of reticence among pharmaceutical companies to run head to head trials of their drugs versus the competition. This is where the US government has stepped in. As with the CATT trial (which compared ranibizumab with bevacizumab in patients with neovascular AMD, [1]), the National Eye Institute (NEI) has funded the DRCR.net’s Protocol T trial, which compared aflibercept, bevacizumab and ranibizumab for the treatment of DME (2).
The study’s chairperson, Jack Wells explains, “The relative effectiveness and safety of the three drugs was unknown. We compared them by randomly assigning 660 patients with center-involved DME and vision loss from 20/32 to 20/320 to receive intravitreous injections of either aflibercept, bevacizumab, or ranibizumab at a maximum of every four weeks for one year. The primary outcome of the study was the mean change in vision at one year in each group.” Was there a clear winner? “The results are reassuring: all drugs have been shown to be effective in improving vision and are equally safe, so doctors can confidently use them,” says Wells. “In eyes with mild vision loss at baseline (20/32 to 20/40), the average gain in vision was eight letters with all three, but in eyes with baseline vision of 20/50 or worse, aflibercept-treated eyes gained significantly more vision – about one line on a Snellen chart – than eyes treated with the other two drugs” (Figure 1).
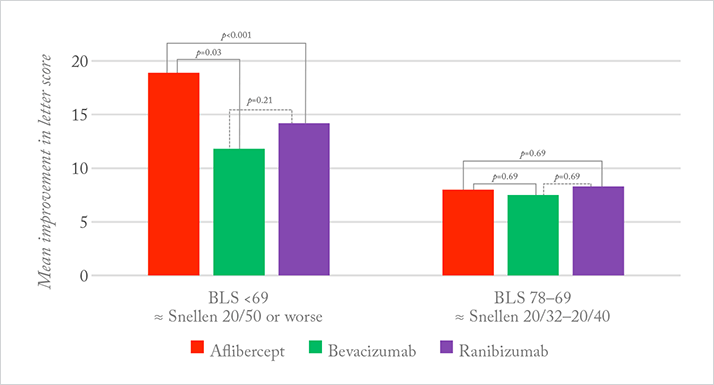
What might be the explanation? According to Wells, there are differences in the structure and relative VEGF binding affinities of the drugs, with aflibercept having the highest binding affinity. A pre-specified hypothesis of the study was that eyes with worse vision would have worse DME and greater macular thickness on OCT – due to higher retinal VEGF levels – and there might be differences in the drugs’ efficacy related to their VEGF binding affinities. “That is why the interaction with baseline vision is compelling: it was pre-specified in the study’s statistical analysis plan,” Wells explains.
References
- DF Martin et al. “Ranibizumab and bevacizumab for neovascular age-related macular degeneration”, N Engl J Med, 364, 1897–1908 (2011). PMID: 21526923. JA Wells et al. “Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema”, N Engl J Med, [epub ahead of print] (2015). PMID: 25692915.
