- There have been many attempts to try to minimize the development of post-cataract surgery PCO, but ~25 percent of patients still go on to develop it
- Nd-YAG laser capsulotomies help address it – but this involves additional costs and is not without risk
- An IOL that combines two PCO-combatting measures – a 360° haptic design, and a membrane that combats lens epithelial cell migration – has been developed
- Preclinical rabbit studies have shown great promise, which if delivered in a clinical setting, could spare millions from Nd-YAG laser capsulotomy over the coming decades
Each year, 22 million people worldwide undergo cataract surgery– and that number is set to rise to 32 million by 2020, according to the World Health Organization. Most of the time, it’s a hugely successful procedure, but for approximately 25 percent of that 22 million, their vision may deteriorate afterwards. Why? Posterior capsule opacification (PCO), which results from the growth and abnormal proliferation of lens epithelial cells (LECs) that were present on the capsule at the time of cataract surgery. The LECs migrate to the posterior capsule where they undergo aberrant differentiation into fiber-like cells or transdifferentiation into fibroblast-like cells, obscuring the central visual axis, causing hazy vision.
It’s not the end of the world – if PCO occurs, it can be treated with a YAG laser capsulotomy, which is effective and relatively quick – but obviously, second surgical procedures are associated with additional costs and risks. The costs are handled differently depending on each country’s healthcare system – in some countries, the YAG laser costs are bundled into the initial cataract surgery; in others they remain separate. In the US, where we’re based, Medicare data shows that the costs of PCO are significant: around $350 million per year, and that’s set to rise to $1 billion by 2050.
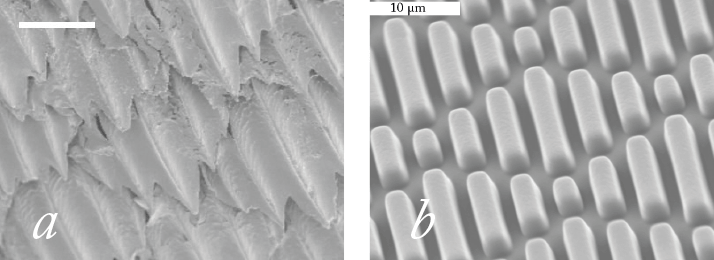
Shark stimulus
What if there were an IOL that could dramatically reduce the incidence of PCO? One way of achieving that would be to have an IOL that’s resistant to LEC migration. Thanks to the landmark work of David Spalton, we’ve known for over a decade that square-edged intraocular lenses (IOLs) exert significantly more pressure on the posterior capsule at the optic edge than round-edged IOLs, forming a barrier against LEC migration and PCO. But as the contemporary PCO rates show: that isn’t enough. What if there were a material that also made it much harder for LECs to migrate? Sharks violate a primary rule of the ocean: things that go fast stay nice and clean; things that move slowly have things growing on them. Sharks move slowly and are clean. The reason why is the physical properties of their skin: the microscopic pattern of shark skin actually inhibits colonization by everything from floating microorganisms to barnacles (Figure 1a). This texture has been replicated and refined, and the resulting pattern – Sharklet (Figure 1b) – is currently doing a great job of inhibiting bacterial migration in medical devices such as endotracheal tubes and venous and urinary catheters. This led us to question if an IOL that replicated this surface pattern could inhibit the LEC migration that causes PCO. To answer it, we developed the ClearSight posterior chamber IOL: a lens that combines a novel 360° square-edged haptic design with a Sharklet-patterned protective membrane (Figure 2).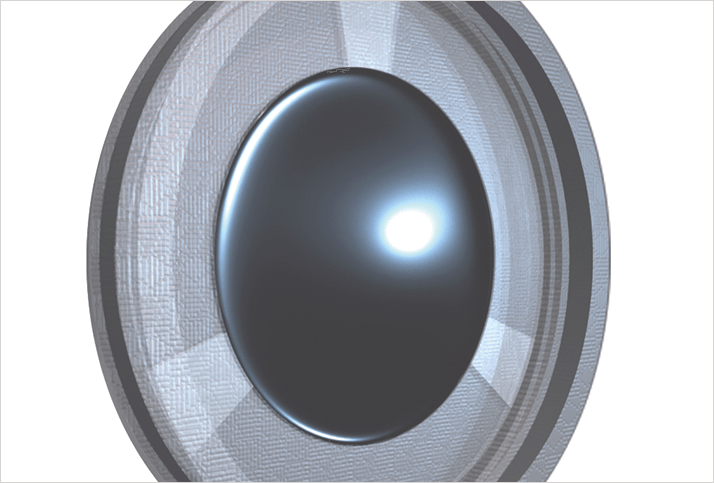
The power of the pattern
First, we tested a number of Sharklet micropatterns in a modified scratch wound assay for their ability to reduce or inhibit human LEC migration relative to a smooth surface control (1). The LECs freely migrated across the smooth surface control, whereas the best performing Sharklet pattern achieved an 80 percent reduction in human LEC migration (Figure 3). The unique discontinuous features that comprise the Sharklet micropattern allow for cells to be precisely guided away from the visual axis using strictly physical stimulation. The best performing topography was selected, translated to a radial design, and applied to IOL prototypes.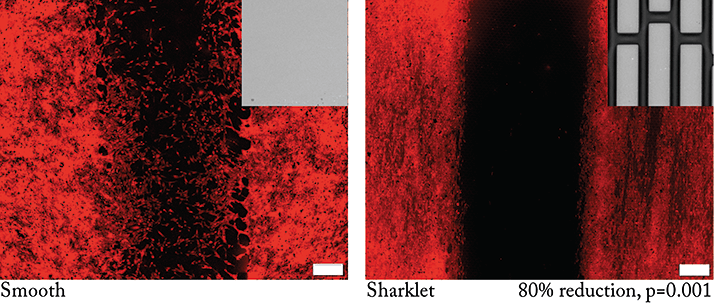
Preclinical validation
Next, the ClearSight IOL prototype was compared with a standard IOL in a rabbit model of PCO formation (2). We observed considerable PCO with the standard IOL, and little to none with the ClearSight IOL – the new design reduced PCO scores by 70 percent in clinical examinations compared with the standard IOL design (Figure 4). Ophthalmologists who were blinded to the treatment group also evaluated slit-lamp exam images and confirmed that none of the ClearSight IOL prototype eyes would require YAG laser capsulotomy treatment. Clearly, the next step is for a clinical evaluation of the ClearSight IOL.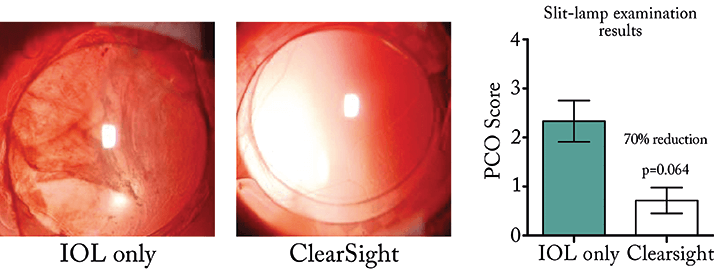
As the 360° square-edged haptics and the Sharklet pattern can be applied to monofocal, multifocal and toric lens designs, it holds the potential to reduce PCO in the vast majority of IOL use cases – and solve one of the most common surgical complications in one of the most commonly performed surgical procedures in the world.
Chelsea Magin is the Director of Product Development at Sharklet Technologies, Inc., Aurora, CO, USA.
References
- CM Magin, et al., “Micropatterned protective membranes inhibit lens epithelial cell migration in posterior capsule opacification”, Tran Vis Sci Tech, 4(2), 1-9 (2015). PMID: 25883876. GD Kramer, et al., “Evaluation of stability and capsular bag opacification with a foldable intraocular lens coupled with a protective membrane in the rabbit model”, J Cataract Refract Surg, [In Press] (2015). AR Vasavada, et al., “Posterior capsule opacification after lens implantation”, Expert Rev Ophthalmol, 8, 141–149 (2013).
