We all know deep anterior lamellar keratoplasty (DALK) is better than penetrating keratoplasty (PK) for treating keratoconus because you’re avoiding the risk of graft rejection. But when you look at certain national registries, you see that only a third of keratoplasties are DALK – and two-thirds are PK. Why? “The FEMTO LDV Z8 femtosecond laser can improve anyone’s DALK technique.” We believe it’s because the big bubble technique used to prepare the DALK graft can be very difficult: the challenge is to make it easier for surgeons to perform. OCT-guided DALK with the FEMTO LDV Z8 is my response to that challenge (Figure 1). Briefly, you can use the Z8’s intraoperative OCT to precisely control the laser when making side-cuts and lamellar/stroma cuts, removing anterior stroma, and constructing a guide channel which terminates close to Descemet’s. Use the channel to direct the cannula so that it ends up next to Descemet’s without penetrating it. Then blow air through the cannula to form a bubble, and complete the procedure per the usual DALK protocol. This isn’t ‘dumbing down DALK’ – the procedure remains challenging – but it is a robust way of improving anyone’s DALK technique. www.femtoldv.com/dalk
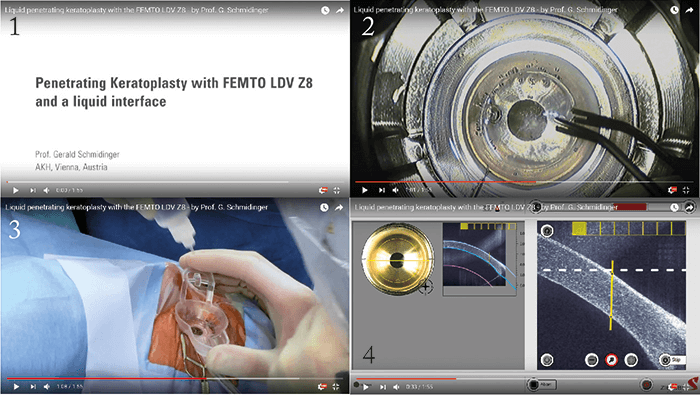
The Z8 is a versatile tool for corneal surgery. It can be used to make intracorneal pockets for items like corneal inlays or intracorneal ring segments, and also (of course) for PK and lamellar keratoplasty procedures like DALK. But what I’d like to discuss are the advantages of using a femtosecond laser over the manual method that most people still perform to trephine both donor and recipient corneas. “Surgeons now can – for the first time – perform non-applanated corneal keratoplasty with a femtosecond laser.” The first advantage is that you should not have an undercut – and in any event, even if you do, you will have the same side-cut geometry in both the recipient and donor corneas, so it will fit well. The second: with the Z8, you can adjust the incision site after docking. The femtosecond laser approach also avoids the risk of tilting the trephine during the laser treatment – something that we know increases postoperative astigmatism greatly. There’s another advantage: when you use a femtosecond laser, you have better endothelial cells on the periphery of the donor button (1,2), as well as more accurate cut depth. But until now, there seemed to be no difference in visual outcomes between manual and femtosecond laser approaches. Why? It’s probably due to astigmatism – postoperatively, it’s more or less the same with both approaches. And this is likely a consequence of applanation – we know that in thin corneas or corneas with very high K readings, applanation leads to distortion which in turn can lead to ovalized cuts of the recipient corneas, especially in patients with keratoconus. What we need is an applanation-free femtosecond laser trephination for both donor and recipient. And that’s exactly what Ziemer has been working to provide with the Z8 (Figure 2). The Z8 incorporates a BSS-filled artificial anterior chamber for the donor and new software for OCT-guided trephination. In our experience, this ‘no-touch’ femtosecond-laser trephination in PK provides donor and recipient cuts which are perfectly matching and completely free of bridges. To perform this super-accurate excision, the OCT scans in a completely different manner to what you’ve seen before: it scans in eight meridians and automatically segments the borders of the cornea and adjusts the depth of the cut – and produces a perfectly round cut. We took this back to the lab to look at Descemet’s folds and the stress on corneal transplants, with and without applanation. We saw that the stress on the donor buttons was significantly less with the liquid interface – and the side cuts were also more reproducible. What does this mean? Surgeons now can perform non-applanated, femtosecond laser corneal transplantation. The Z8 liquid interface reduces stress on donor buttons, with significantly less Descemet’s folds, and provides cut geometries that are more exactly matching. www.femtoldv.com/liquidpkp
One of the misconceptions about femtosecond laser system use in cataract surgery is that it will slow patient workflow. My response is: not if you’re doing it right. We have used the Z8 for over three years and we have found it to offer benefits including (3): less phaco time; repeatable outcomes; and better visual acuity on post-operative day 1. Furthermore, the Z8’s OCT imaging system permits customized treatment, which is a huge advantage. But what about workflow? When we started, FLACS procedures with the Z8 did take significantly longer than manual cataract surgery – but we eliminated that difference by making simple changes to our workflow. First, the nurse prepares the laser for the next patient while the surgeon concludes the current procedure; this alone reduced the time for preparing the laser from 3.2 minutes to ~2.1 minutes (a ~30 percent reduction). Second, we get the next patient in the operating room within 30 to 50 seconds of the previous patient leaving. Our current Z8 FLACS turnaround time is in the region of 7–8 minutes per patient, which translates to five to six procedures per hour. So the Z8 gives you all of the advantages of FLACS, like optimal capsulotomy placement and improved OCT-based surgical planning with no added time.
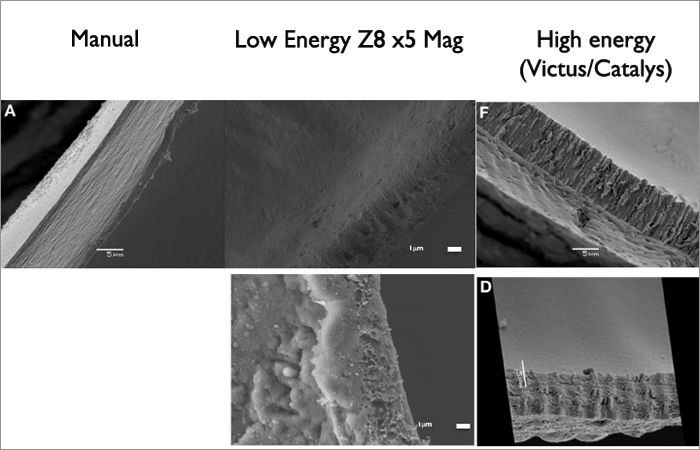
The Z8 contains a number of superb technical innovations: a liquid-optic interface, nanojoule energy, high-frequency pulses and the option of both vertical firing (for lens fragmentation) and rotational firing (for capsulotomies). But do these features give patients better outcomes? Let’s start with capsulotomy strength. Many studies have shown equivalent results to manual capsulorhexis – but most have been performed in pig eyes, some with the lens in, some with the lens out, and there’s been great variation between the femtosecond laser platforms used to create the capsulotomy. We compared Z8 capsulotomies with manual capsulorhexes in porcine and human eyes (4). Interestingly, we found that the rhexis got stronger with increasing capsulotomy size – a 5.5 mm rhexis is stronger than a 5 mm rhexis. Furthermore, compared to the Victus and Catalys systems, the Z8’s rotational firing created a very smooth edge, similar to manual capsulorhexes. The vertical firing patterns of high energy lasers, however, score the tissue; this may cause tags, capsular tears, and capsular distortion following IOL implantation. But pig eyes are obviously different to human eyes – and their capsular bag is very elastic, almost like pediatric eyes. Further, the stretch ratio is better in human than pig eyes, which is important when you’re looking at capsulotomy strength. But what’s interesting is the scanning electron microscope comparison of the cuts made with the Z8 and other femtosecond lasers: the nanojoule pulse Z8-cut surface is smooth, like a manual rhexis, whereas higher energy lasers like the Victus and Catalys cause ridges (Figure 3).We believe this is why some people experience tags with the latter systems that can lead to runaway anterior capsular ruptures. “With the Z8, cell death rates were basically the same irrespective of whether 90 or 150 percent energy was used, completely different from other femtosecond laser systems.” But what about eyes with thicker corneas – like those that have undergone a prior DALK procedure? To understand the principles of lasering in eyes with thick corneas, we went back to study pig eyes with corneas in the range of 650–1,000 µm and pushed the laser to the limits. Regular laser energy use (90 percent) sometimes resulted in nice capsulotomies, but often didn’t – sometimes you saw some very odd laser patterns due to the dispersion of the laser energy through the thickened cornea. When we increased the energy from 90 to 130 or even 150 percent, even with corneal thicknesses of 1,000 µm, we got a perfect capsulotomy every time. But this could raise a potential problem. We know in other laser systems (LenSx and LensAR) that increased energy weakens the capsulotomy, and may even melt the edge! Hence we wanted to study the effect of higher nJ on the lens capsule. We found that with the Z8’s nanojoule laser system, the capsulotomy stretch ratios remained the same, irrespective of whether the laser was used at 90, 130 or 150 percentage energy. If we looked at the standard deviation of the stretch ratios, we found there was a much tighter spread with 150 percent energy than with 90 percent. In other words, the Z8’s capsulotomy was still strong, even with 150 percent energy and a corneal thickness of 1,000 µm. When we took these settings to patients, we saw the same story, and on removing the capsules and performing a cell death assay in the lab, we found that cell death rates were basically the same, irrespective of whether 90 or 150 percent energy was used – which is a completely different story to that found with other high pulse energy femtosecond laser systems (5).
We know that FLACS with other higher energy femtosecond lasers can result in elevated levels of prostaglandins and inflammatory cytokines, a rise in anterior chamber temperature of up to 5°C and a reduction in pH – and these can lead to CME, ocular hypertension and intraoperative miosis (6)(7). However, the Z8 employs unique aperture geometry to emit pulses of low energy (nanojoule) and high frequency (in the megahertz range); it delivers only 1.5 percent of the energy per pulse of other manufacturers’ femtosecond laser platforms, and this favors the creation of smooth cuts that are free of tears or tissue bridges. But is this reduced energy reflected in lower inflammatory mediator production during cataract pretreatment? We performed a randomized intra-individual comparison study where aqueous humor samples from Z8-treated eyes taken 5 minutes after laser docking were compared with those from manually-treated partner eyes (40 cataract patients). We found no significant difference in the concentrations of the inflammatory mediators interleukin-6 and -1β.
It’s becoming increasingly apparent that femtosecond lasers – and in particular the Z8 – are extremely useful when it comes to treating complex cataract cases, like shallow anterior chambers, white (but not intumescent) cataracts, brown nuclei, zonular weakness, low endothelial cell counts, and even pediatric cataracts. Let’s look at pediatric surgery. The Z8 allows us to create the main and side port incisions and perform perfectly sized and well-centered capsulotomies of children’s eyes all in a single environment, without ever needing to move the patient. Indeed, a great advantage of the Z8 workflow is that the surgeon is the center of the procedure, and we no longer need to move the patient to the laser. In one case, I found the Z8 to be of great assistance in creating a smooth capsulotomy in a child with a traumatic cataract who had vitreous strands in the anterior chamber. The Z8’s OCT imaging system permitted visualization of critical features, enabling capsulotomy even though vitreous was in the anterior chamber. “A great advantage of the Z8 workflow is that the surgeon is the centre of the procedure, and we no longer need to move the patient to the laser.” Further, the Z8 helps in providing more reproducible posterior capsulotomies, which is specifically useful in pediatric cases when the PCO rates are close to 100 percent. Laser posterior capsulotomy may help to facilitate a bag-in-the-lens approach. In cases of zonular weakness, the Z8 allows the creation of capsulotomies without stressing the zonules. The Z8’s OCT facility is extremely helpful in dealing with cases of ectopic lenses – the surgeon can assess and adjust the parameters of the femtosecond laser to deal with tilted (or non-tilted) ectopic lenses to produce a superb capsulotomy (Figure 4). The Z8’s OCT is also extremely useful when dealing with patients with peripheral corneal scars such as in post-radial keratotomy eyes. The laser may be blocked by the scar tissue, but it can create a perfect partial capsulotomy (aimed with the OCT), such that the surgeon can follow the contour of the cut and easily complete the procedure with forceps. Finally, the Z8 can be useful in bi-lensectomies: it not only creates the capsulotomy but also can be used to chop the phakic intraocular lens so that it can be removed without extending the incision. So even if the advantages of FLACS over conventional cataract surgery are still being debated, I believe that the Z8 can have a very broad impact in a variety of complex cataract cases.
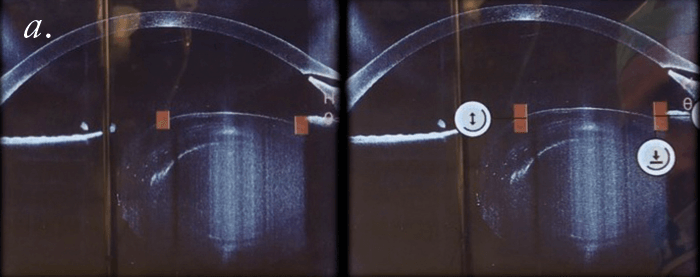
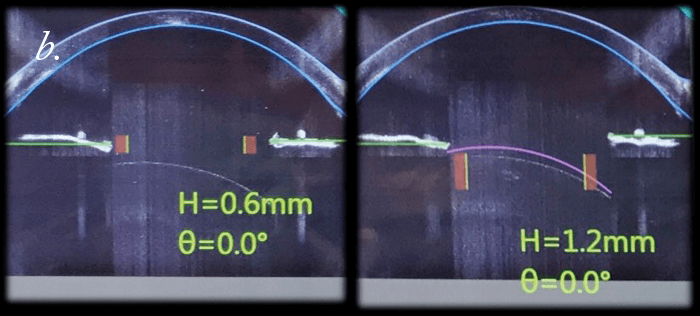
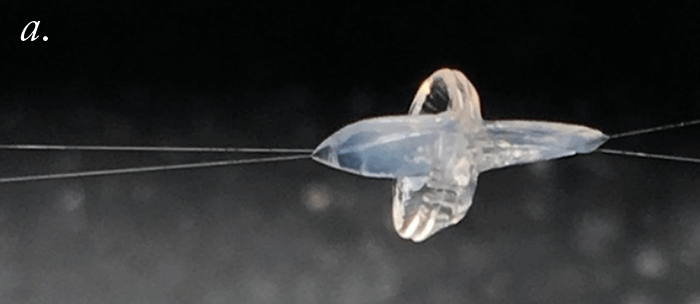
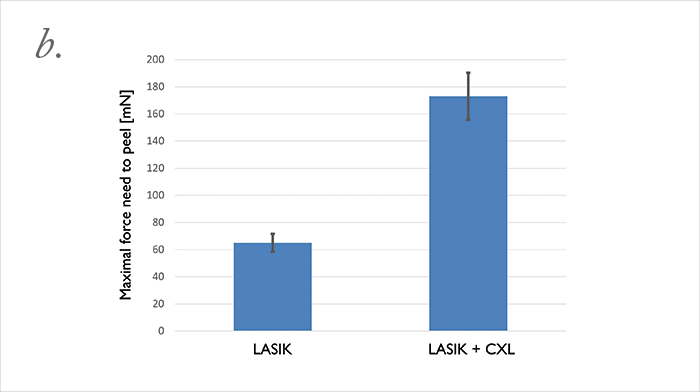
Can we improve the safety of LASIK? Well, we did a study to find out more. We used the FEMTO LDV Z6 to create LASIK flaps in rabbit eyes; then we applied riboflavin, reattached the flap to the stromal bed, and performed UV-A irradiation to cross-link. When we do this clinically, we noticed that in some cases re-lifting the LASIK flap is almost impossible. We performed adhesion measurements on the rabbit cross-linked flap (it’s essentially a shear experiment, where we shear the flap against the stromal bed). We optimized the procedure in terms of riboflavin concentration and irradiance exposure, and were able to achieve a bonding of the flap to the stroma that was two times stronger than riboflavin-only (or LASIK flap only) controls. We then asked what the effect was over time: at three months of follow-up, we attempted the shear experiments again. It was impossible to shear the flap; the flap was stuck to the stroma so strongly that we ended up having to suture the flap on one side, and the stromal bed on the other, then pull them apart (Figure 5a) – the cohesion, instead of being two times stronger than control flaps, was now three times stronger! If you’re using LASIK with CXL clinically, be aware that this combination of techniques will mean that patients have slower visual rehabilitation and that you might also induce early post-operative complications like erosions or diffuse lamellar keratitis. Nevertheless, we can conclude that the combination of LASIK and CXL seals the interface between the flap and stromal bed, and looks like it should decrease the risk of central (but not peripheral) iatrogenic corneal ectasias – and improve the safety of an already extremely safe technique even further.
References
- RI Angunawela, et al., Invest Ophthalmol Vis Sci, 53, 2571–2579 (2012). PMID: 22427557. Kim JH, et al, Cornea, 28, 812–816 (2009). PMID: 19574902. B Pajic, Z Cvejic, B Pajic-Eggspuehler. Sensors, 17 (2017). PMID: 28629164. P Williams et al., Sci Rep, 6, 24352 (2016). PMID: 27090745. WJ Mayer et al., Invest Ophthalmol Vis Sci., 55, 893–898 (2014). PMID: 24408981. Schultz et al., J Refract Surg, 29, 742–747 (2013). PMID 24203805. R Yeoh, J Cataract Refract Surg, 40, 852–853 (2014). PMID: 24767932.
