- The biggest practical drawback of intravitreal anti-VEGF injections is their frequency
- Reducing the injection burden is a significant unmet need for patients with retinal vascular disease
- A number of approaches might solve the problem – and if any one of them does, it’s a game-changer
- Here, I review the latest developments in long-acting retinal drug delivery formulations
We now accept that neovascular AMD is best treated by fixed intravitreal doses of anti-VEGF drugs; this approach gives the best responses in terms of visual acuity and deviating from it with as-needed treatment regimens results in inferior outcomes. However, patients dislike intravitreal injections, and the frequency of those injections (generally monthly or bimonthly) is burdensome to doctor and patient alike. In short, everyone would prefer fewer injections. One strategy is to employ sustained drug release formulations, and some of the approaches currently under investigation include biodegradable polymers, lipid-based delivery, reservoir implants, gene therapy, encapsulated cell technology, and choroidal depot delivery. The success of any of these approaches will likely transform the treatment of macular degeneration. So what is the current state of play?
Polymer matrix formulations
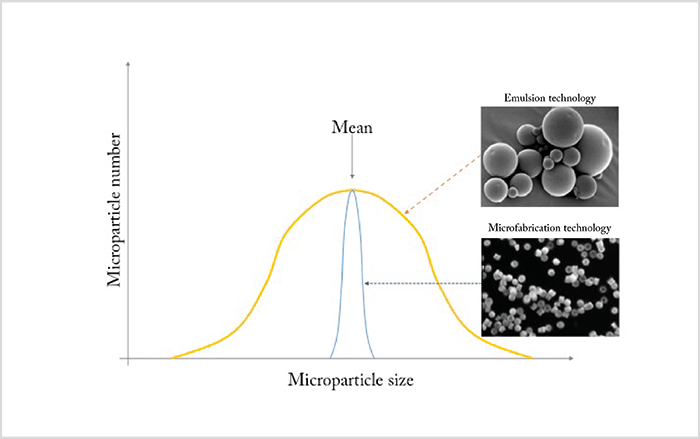
Loading a drug into a polymer matrix from which it slowly escapes by diffusion as the polymer biodegrades is a well-established drug delivery strategy. The most commonly used polymer backbones are based on repeating polylactides, like poly-lactic-co-glycolic acid (PLGA). These polymers – manufactured as spheres or rods of nanometer to micrometer dimensions – are loaded with drug, injected into the eye, and continue to release the drug until the polymer matrix has biodegraded and is resorbed. In the past, these particles were produced using emulsion technology, a method that generates particles of widely varying sizes (Figure 1). Unfortunately, this size variation means that the surface area:volume ratio is different for every particle – as is the drug release kinetics. It’s therefore preferable to generate particles of uniform dimensions. Fortunately, the semiconductor industry has solved this problem – microfabrication technology now enables manufacturers to create a template that gives them tight control of particle size – and with it, control of the drug release characteristics of the final product (Figure 1). The approach looks promising; initial studies with microfabricated PLGA particles loaded with anti-VEGF agents show steady drug release over four months (1)(2). Furthermore, microfabrication technology supports the manufacture of more sophisticated structures, such as multi-layered particles, which opens up the possibility of loading single multi-layered particles with different drugs that are released at different times – as has recently been exploited to good effect with single-dose combination vaccines that deploy at different time points (3). Further flexibility can be achieved through careful choice of delivery vehicle. For example, drug-loaded particles can be suspended in a solution of the drug itself, providing an initial ‘burst’ effect at administration, along with the chronic and sustained drug release provided by the particles. Another possibility is to suspend the drug particles in a gel-forming polymer. Here, thermoresponsive hydrogels show great promise – they remain liquid at room temperature, but solidify at body temperature. This means that you could inject a liquid therapeutic agent that becomes a gel soon afterwards – ideal for depot-type sustained release systems. By way of an example, a hydrogel containing ranibizumab particles was shown to release the drug over a period of at least 210 days from one injection (Figure 2).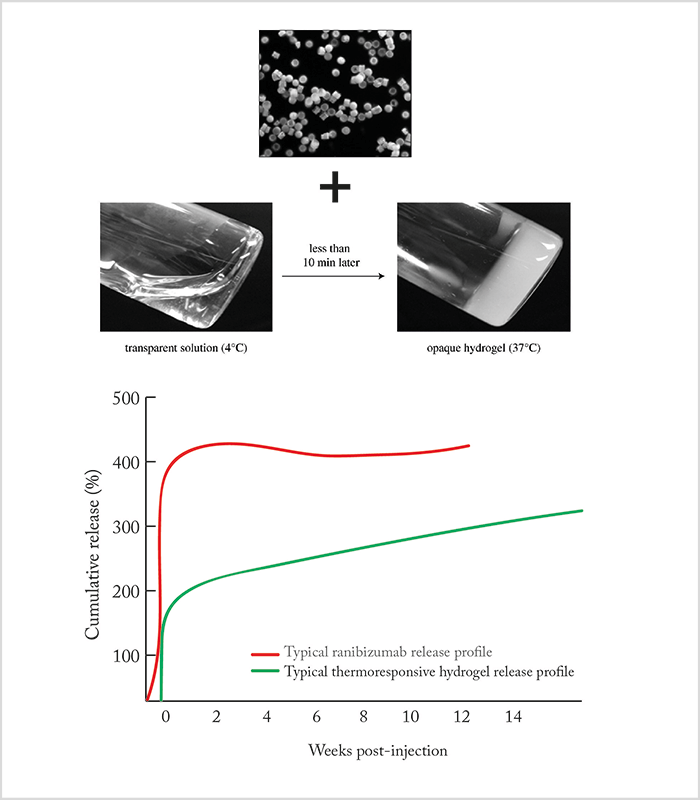
Lipid-based delivery
Assembling multi-laminar liposomes in the presence of drug, such that the lipid layers form drug-loaded compartments, offers another approach; as the lipids break down, the drug is released (Figure 3). The kinetics of drug release from the liposomes, including duration of drug release, can be modified by adjusting the phospholipid formulation.Reservoir implants
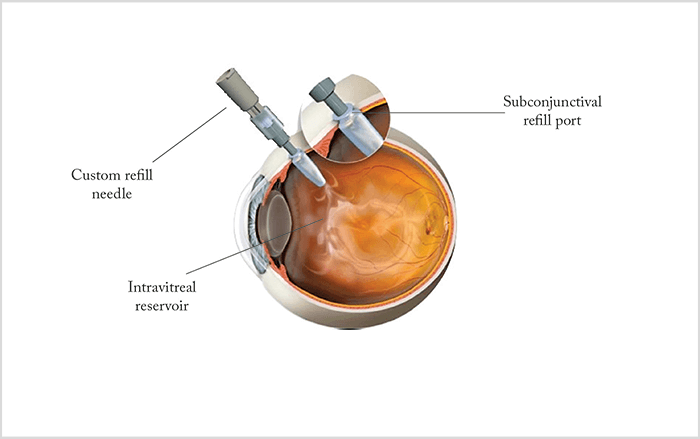
Mechanical drug delivery devices have also attracted much interest. One example is the Replenish intraocular pump (see Box below), which comprises an in-office refillable drug reservoir attached to a programmable micropump that enables controlled ocular drug delivery for as long as nine months. The micropump can be both recharged and reprogrammed wirelessly, permitting its functions to be fine-tuned as required without physical external access. Similarly, a port delivery system allows infusion from an intravitreally-positioned drug reservoir. The device is implanted surgically, using a 3.2 mm scleral incision without sutures, and slowly releases the drug over 3–4 months (Figure 4). At the end of this period, the reservoir can be refilled via the subconjunctival port; this step requires only a minimally invasive in-office procedure. A ranibizumab port delivery system is currently under Phase II clinical evaluation (NCT02510794).- Delivers injections hourly, daily or monthly
- Large reservoir capacity:
- Refillable by in-office procedure using proprietary 31 G needle system
- Dose can be modulated by wireless programming
- Battery recharged by wireless induction charging
Gene therapy
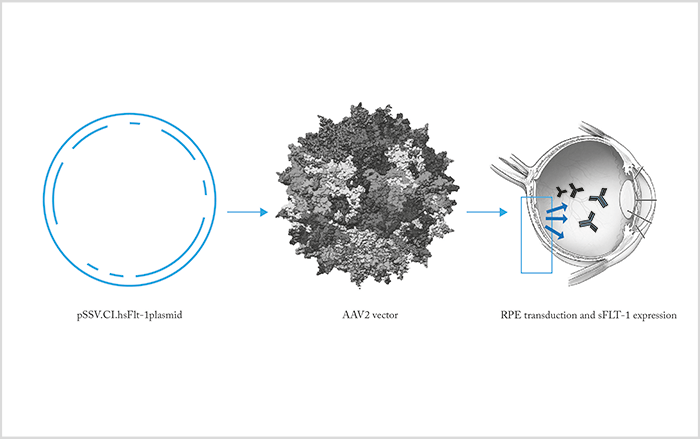
Gene therapy offers the hope of permanent intraocular drug release by exploiting the protein synthesis machinery of ocular cells (4). Genetic modification of ocular tissue is effected by means of vectors, such as replication-deficient adenovirus, carrying DNA that encodes a therapeutic protein that is expressed by the target cells. Modes of administration of ocular gene therapy include intravitreal injection and subretinal injection of vectors – the approach varies by the retinal cell type you wish to transfect. For example, an adenoviral vector has been used to transduce cells with material encoding a chimeric protein, sFLT-1, that can bind and block both VEGF and PLGF. Similarly, Avalanche Biotech has used a vector based on adeno-associated virus to transduce RPE cells such that they express sFLT-1 protein. This approach resulted in strong sFLT-1 expression over the entire area of the subretinal injection (Figure 5). However, phase 2 studies were not successful. RegenexBio is looking at even better AAV vectors to deliver anti-VEGF plasmids.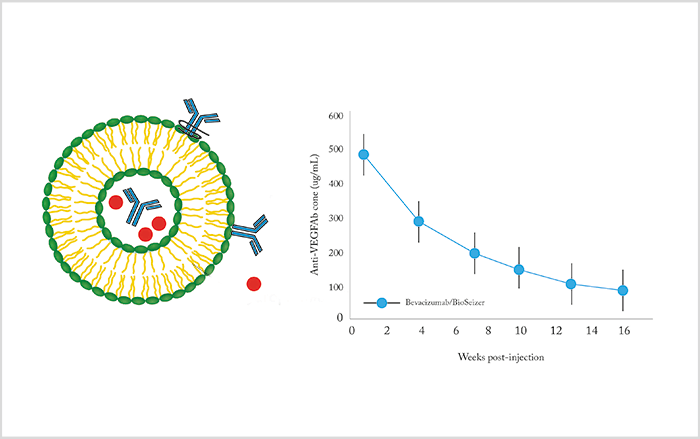
Cell therapy
Encapsulated cell technology (ECT) involves implantation of devices containing populations of living cells that secrete a therapeutic protein (5). These cells are separated from host tissues by a semi-permeable implant wall, which protects the encapsulated cells from the patient’s immune system while permitting inward diffusion of oxygen and nutrients and outward diffusion of therapeutic protein(s). At present, ocular ECT strategies use a proprietary cell line derived from human RPE cells; the cell line has been selected to be non-tumorigenic, viable under nutrient-poor / oxygen-poor conditions and capable of producing clinically relevant levels of therapeutics in vivo. The normal form of the ECT device is a cylinder, which is surgically implanted into the eye and tethered to the sclera by a titanium clip (Figure 6). Since drug release is correlated to surface area, adequate doses – for example, 10–12 µg/day – may require multiple cylinders to be implanted per eye. One example of this technique comprises human RPE cells that have been genetically engineered, by plasmid transfection, to secrete sFLT1.Choroidal depots
We can also exploit the natural anatomy of the eye for drug delivery purposes (6). In particular, we can use the choroid as a depot; administration of drug can be achieved by cannulating the suprachoroidal space and injecting drugs either with a microcatheter or by using microneedles with lengths of between 800 and 1000 µm. Top-ups are then a matter of injecting drugs through these devices into the suprachoroidal space, from where the therapy can diffuse into the eye.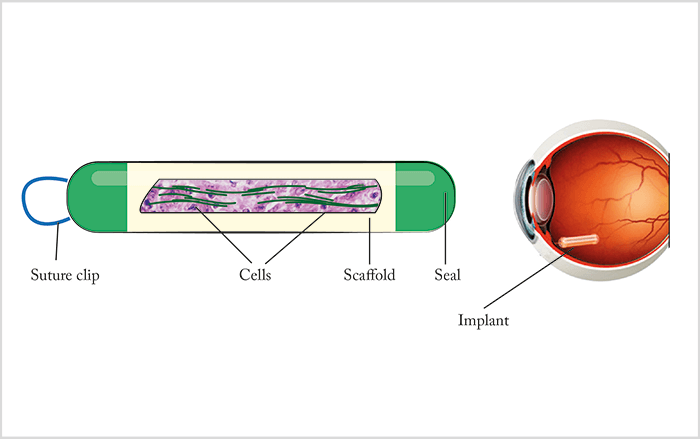
Keeping hopes high
The injection frequency associated with the current AMD standard of care is widely recognized to be problematic, which has led to a number of technical innovations aimed at improving ocular delivery of biotherapeutics. Sustained-release solid implants are the most advanced right now, but it is still too early to say which of the various procedures under development will have the most impact in AMD. No matter who the victor, I hope that we will be able to offer our macular degeneration patients an alternative that demands far fewer injections in the near future. Keep watching this space! Peter Kaiser is Professor of Ophthalmology, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and Senior Vice President of Product Development at Ohr Pharmaceutical. He is a consultant for Alcon, Allegro, Allergan, Aerpio, Bayer, Biogen, Digisight, Genentech, Neurotech, Novartis, Ohr Pharmaceuticals, Regeneron, Santen, Shire and Thrombogenics, and is both a consultant and stockholder in InSitu Therapeutics, Neurotech, and Ohr Pharmaceuticals.References
- SK Yandrapu et al., “Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab”, Mol Pharm, 10, 4676–4686 (2013). PMID: 24131101. F Li et al., “Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration”, Open Ophthalmol J, 6, 54–58 (2012). PMID: 22798970. KJ McHugh et al., “Fabrication of fillable microparticles and other complex 3D mechanisms”, Science, 357, 1138–1142 (2017). PMID: 28912242. J Bennett et al., “Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina”, Proc Natl Acad Sci USA, 96, 9920–9952 (1999). PMID: 10449795. K Kauper et al., “Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases”, Invest Ophthalmol Vis Sci, 53, 7484–7491 (2012). PMID: 23049090. E Moisseiev et al., “The suprachoroidal space: from potential space to a space with potential”, Clin Ophthalmol., 10, 173–178 (2016). PMID: 26869750.
