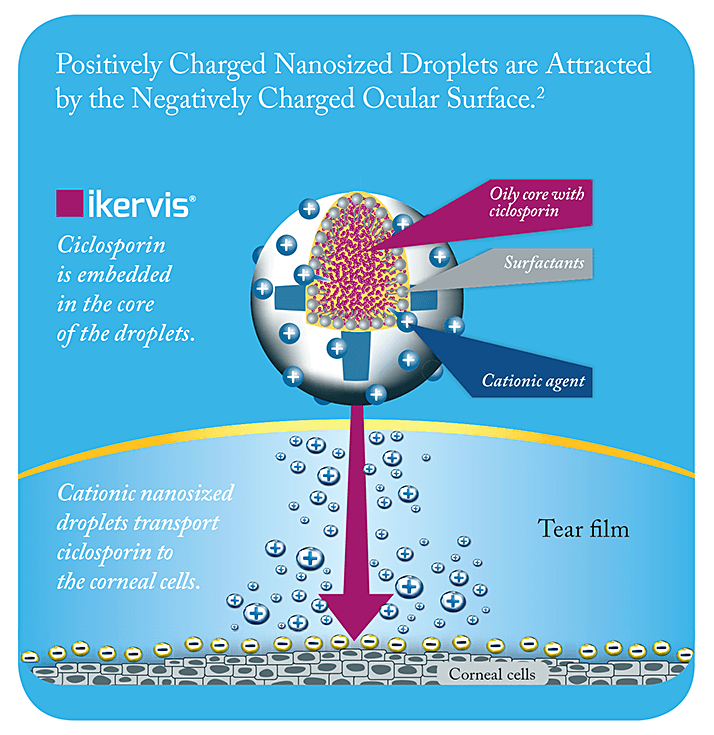
There’s currently a considerable unmet need amongst people with severe dry eye disease (DED): obtaining effective treatment (1). Diagnosis can be challenging when a significant proportion of patients with DED present with discordance between signs and symptoms (2) although thankfully, improved diagnostic algorithms and questionnaires are helping to identify more of those patients with the severe forms of the disease who are in need of an intervention (3). But what are those interventions? Unlike milder forms of DED that can be managed with tear substitutes, lubricant drops or gels for symptom relief, more severe forms of DED are driven by a vicious circle of inflammatory processes that need something more than artificial tears to dampen the disease (4). Without causal treatment, this vicious circle can lead to severe damage to the corneal epithelium (5). Corticosteroids can perform that function, but have a poor side effect profile (especially with chronic use), and risk raising patients’ intraocular pressure or inducing cataract formation (6). The other way to dampen inflammation is to use ciclosporin-containing eyedrops (7), but this has been a challenge in the EU so far due to the absence of an approved, commercially available product. That was yesterday’s challenge. Ikervis, 1 mg/mL ciclosporin (Santen) has recently received marketing approval in the EU for the “treatment of severe keratitis in adult patients with dry eye disease, which has not improved despite treatment with tear substitutes” (8–10). The posology is simple: a single drop into each affected eye once daily at bedtime (8). Ikervis has a three-year shelf-life, is supplied in single-dose containers (8), and Ikervis is now approved for use in the EU. The formulation is worth a closer look. Ikervis is a cationic oil-in-water emulsion of ciclosporin, based on Santen’s Novasorb technology (11). The positively charged nano-sized droplets of the emulsion electrostatically adhere to the negatively charged mucins on the ocular surface, thereby improving ocular retention and absorption (Figure 1), and the lipids in the formulation support the stabilization of the tear film, too (11). The fact that the droplets are nano-sized is important: as droplet size reduces, the surface area to volume ratio increases, meaning a greater total surface area of the emulsion is exposed to the ocular surface – in essence, the eye “sees” more of the eyedrop. This has been demonstrated in rabbits (12) – Ikervis (1 mg/mL ciclosporin) administration results in corneal ciclosporin concentrations around four times greater than that of an anionic emulsion of 0.5 mg/mL ciclosporin. That is why the innovative cationic formulation of Ikervis makes once-a-day dosing possible (8).
Ikervis’ regulatory approval was based on the results of its clinical trial program, which enrolled 982 patients in total. All studies were multicenter, randomized, controlled, double-blinded comparisons of ophthalmic cationic emulsion of ciclosporin versus vehicle control, i.e., versus cationic emulsion without any pharmaceutically active ingredient (9,13). SANSIKA, the pivotal phase III study, was a 12-month trial that enrolled 246 DED patients with severe keratitis (defined as a corneal fluorescein staining [CFS] score of 4 on the modified Oxford scale). SICCANOVE was a 6-month trial that enrolled 492 DED patients with moderate to severe keratitis (CFS score of 2 to 4). Both studies showed that treatment with Ikervis was associated with significant improvements in CFS from baseline values and versus vehicle control, and an exploratory analysis in SANSIKA performed at month 6 found that ocular surface inflammation (as determined by human leukocyte antigen-DR expression) was significantly lower (p=0.021) in Ikervis-receiving patients than those receiving the vehicle control (10). In general, Ikervis is safe and well-tolerated – even with long-term treatment. But there are two things patients should be informed about. The active ingredient, ciclosporin, gives rise to the therapy’s most common side effect: eye pain or irritation (8) – often described by patients as a burning sensation upon instillation. However, this is normally mild-to-moderate in intensity (8). Secondly, patients need to be aware that it will take some time before their ocular surface disease starts to improve – Ikervis’ effects aren’t immediate, but over the longer term, it’s effective in reducing ocular surface damage and inflammation, and may prevent DED from worsening (10). Ultimately, the introduction of Ikervis onto the European market means three things. First, unlike artificial tears and lubricants, Ikervis addresses the underlying inflammatory processes present in severe keratitis in DED. Second, its formulation requires no refrigeration (since it can be stored at room temperature) and has a three-year shelf-life (8). And finally, it is the only approved topical formulation of ciclosporin available commercially in Europe today – bringing a novel treatment option to patients with severe keratitis in DED who, until now, had no access to this treatment option.
- PA Asbell, S Spiegel, “Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey”, Eye Contact Lens, 1, 33–38 (2010).
- ME Johnson, “The association between symptoms of discomfort and signs in dry eye”, Ocul Surf, 7, 199–211 (2009).
- C Baudouin, et al., “Diagnosing the severity of dry eye: a clear and practical algorithm”, Br J Ophthalmol, 98, 1168–1176 (2014).
- DEWS. “Diagnostic methodology of the International Dry Eye WorkShop”, Ocul Surf, 5, 108–152 (2008).
- C Baudouin, “The vicious circle in dry eye syndrome: a mechanistic approach”, J Fr Ophtalmol, 3, 239–246 (2007).
- SC Pflugfelder, “Antiinflammatory therapy for dry eye”, Am J Ophthalmol, 137, 337–342 (2004).
- E Donnenfeld, SC Pflugfelder, “Topical ophthalmic cyclosporine: pharmacology and clinical uses”, Surv Ophthalmol, 54, 321–328 (2009).
- IKERVIS 1 mg/mL eye drops, emulsion. Summary of product characteristics. Available at: http://bit.ly/ikervis. Accessed May 15, 2015.
- European Medicines Agency. IKERVIS Public Assessment Report. EMA/CHMP/473489/2014. Available at: http://bit.ly/ikervisEPAR. Accessed May 15, 2015.
- European Medicines Agency, EPAR summary for the public, EMA/56994/2015. Available at: http://bit.ly/ikervisEPARsummary. Accessed May 15, 2015.
- F Lallemand, et al., “Successfully Improving Ocular Drug Delivery Using The Cationic Nanoemulsion, Novasorb”, J Drug Delivery, 2012:604204 (2012).
- P Daull, et al., “Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits”, Cornea, 32, 345–54 (2013).
- RR Buggage, et al., “The effect of Cyclokat (unpreserved 0.1% cyclosporine cationic emulsion) on corneal involvement in patients with moderate to severe dry eye disease participating in a phase III, multicenter, randomized, controlled, double-masked, clinical trial”, Eur J Ophthalmol, RFCOR-115, SOE, Geneva, Switzerland (2011).
References
- PA Asbell, S Spiegel, “Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey”, Eye Contact Lens, 1, 33–38 (2010). ME Johnson, “The association between symptoms of discomfort and signs in dry eye”, Ocul Surf, 7, 199–211 (2009). C Baudouin, et al., “Diagnosing the severity of dry eye: a clear and practical algorithm”, Br J Ophthalmol, 98, 1168–1176 (2014). DEWS. “Diagnostic methodology of the International Dry Eye WorkShop”, Ocul Surf, 5, 108–152 (2008). C Baudouin, “The vicious circle in dry eye syndrome: a mechanistic approach”, J Fr Ophtalmol, 3, 239–246 (2007). SC Pflugfelder, “Antiinflammatory therapy for dry eye”, Am J Ophthalmol, 137, 337–342 (2004). E Donnenfeld, SC Pflugfelder, “Topical ophthalmic cyclosporine: pharmacology and clinical uses”, Surv Ophthalmol, 54, 321–328 (2009). IKERVIS 1 mg/mL eye drops, emulsion. Summary of product characteristics. Available at: http://bit.ly/ikervis. Accessed May 15, 2015. European Medicines Agency. IKERVIS Public Assessment Report. EMA/CHMP/473489/2014. Available at: http://bit.ly/ikervisEPAR. Accessed May 15, 2015. European Medicines Agency, EPAR summary for the public, EMA/56994/2015. Available at: http://bit.ly/ikervisEPARsummary. Accessed May 15, 2015. F Lallemand, et al., “Successfully Improving Ocular Drug Delivery Using The Cationic Nanoemulsion, Novasorb”, J Drug Delivery, 2012:604204 (2012). P Daull, et al., “Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits”, Cornea, 32, 345–54 (2013). RR Buggage, et al., “The effect of Cyclokat (unpreserved 0.1% cyclosporine cationic emulsion) on corneal involvement in patients with moderate to severe dry eye disease participating in a phase III, multicenter, randomized, controlled, double-masked, clinical trial”, Eur J Ophthalmol, RFCOR-115, SOE, Geneva, Switzerland (2011).
