- Integrins mediate retinal neovascularization and form part of the link between the vitreous and the retina
- Integrin antagonism offers an attractive new therapeutic target
- An integrin antagonist, ALG-1001, is currently undergoing clinical evaluation for the treatment of wet AMD, DME and VMT
- ALG-1001 has the potential to complement and/or replace current therapies
The role of vascular endothelial growth factor (VEGF) in retinovascular diseases is well understood. VEGF inhibitors such as bevacizumab, ranibizumab and aflibercept have revolutionized treatment of these diseases over the past decade, but there remain many patients who don’t respond adequately to anti-VEGF therapy. The blockade of a different set of molecules, the integrins, may hold the key to resolving these therapeutic challenges. Integrins are surface receptors that regulate the interaction of cells with other surrounding cells and tissues. They play important roles in cell adhesion, proliferation, shape and motility.
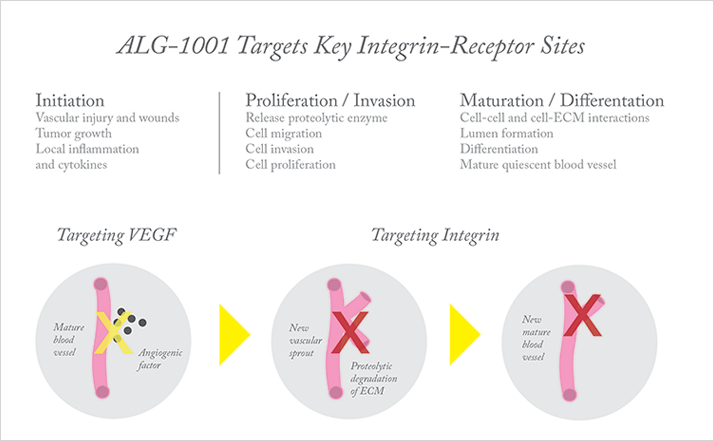
Allegro Ophthalmics’ ALG-1001 is the first drug of a new therapeutic class: integrin peptide therapy. The drug targets integrin receptors at multiple sites. Integrins αvβ3, αvβ5, and α5β1 have been shown in the literature over the past 20 years to be closely associated with both pre-retinal neovascularization (as seen in diabetic retinopathy and macular edema) and choroidal neovascularization (as seen in wet macular degeneration). ALG-1001 specifically inhibits these receptors’ effects on angiogenesis. For example, it can inhibit further growth of abnormal blood vessels, turn off production of new blood vessels, and cause leakage from existing neovascularization to dry up. Furthermore, ALG-1001 activity in binding the integrin α3β1 receptor sites at the vitreoretinal interface facilitates the release of cellular adhesion between the vitreous and the retina, inducing posterior vitreous detachment (PVD).
Integrin peptide therapy operates by a mechanism of action that is different from that of anti-VEGF agents (Figure 1). If we imagine neovascularization as a factory that produces blood vessels, anti-VEGF drugs act as a signaling switch that tells the factory’s machinery to shut off. Inhibition of the integrin pathway is more akin to sabotaging various pieces of machinery – so that even if they are turned on, they can no longer function properly. All the machines downstream in the manufacturing process are starved of product to work on. So in the eye, integrins on the “bud” or leading edge of an existing blood vessel may be prevented from initiating the proteolytic changes that break down surrounding tissues and permit tissue penetration by the new blood vessel. Because of their different mechanisms of action, the integrin and VEGF pathways likely offer complementary – and potentially synergistic – approaches to tackle pathological angiogenesis in the retina.
Early and Mid-Phase Trial Results
Clinical trials of ALG-1001 are progressing in several therapy areas where the compound has the potential to complement or replace current therapies, including wet age-related macular degeneration (AMD), diabetic macular edema (DME), and vitreomacular traction (VMT). What has been most encouraging in the initial results from these trials is that they have demonstrated a high degree of efficacy and a long duration of action.Wet macular degeneration
A high bar for treatment of wet AMD has been set by anti-VEGF drugs; in most cases, they work. However, one of the major challenges with current therapy is the frequency of injections required. They are unpopular with patients, and consume healthcare resources and the time of the patient. Additionally, a sizeable number of patients either do not gain functional vision following anti-VEGF therapy or respond initially but, over time, continue to develop fibrosis and an enlarging central vision defect. The questions for integrin therapy are:- Will it work at least as well as existing therapies?
- Will the duration of effect be longer than current therapeutic options?
- Can it improve outcomes in patients currently plateaued or unresponsive to existing therapies?
Allegro performed a phase Ib/IIa monotherapy study that was designed primarily to evaluate the safety and optimal dosage of ALG-1001. The drug was administered to twenty-two patients with wet AMD. Patients received three monthly injections of ALG-1001 (at doses of either 2.0 mg or 3.2 mg) in monotherapy after a six-week washout period from anti-VEGF therapy, and were then followed-up for four months, off-treatment. The higher dose proved more effective, so a 5.0 mg dose, for which data are still being collected, was added to the study. The peak average improvement in best-corrected visual acuity (BCVA) with the 3.2 mg dose was 5.2 letters (Figure 2). This was accompanied by a 30 percent mean improvement in central macular thickness measured on OCT. The gains were maintained throughout the four months of follow-up, and as long as six months in some subjects, implying that there is a long-acting aspect to this drug which could reduce treatment burden.
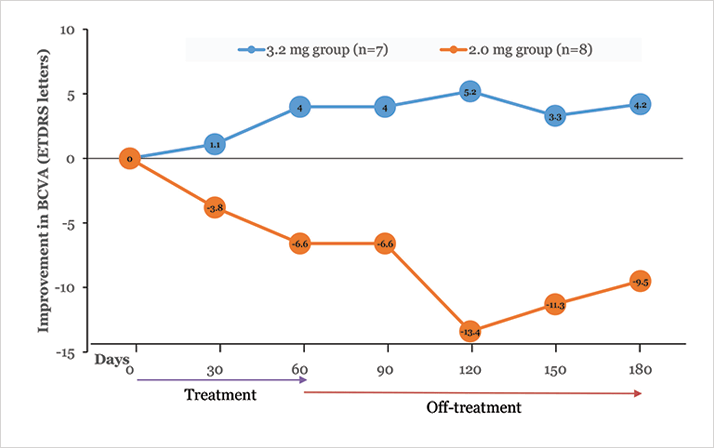
Allegro have received clearance to commence a larger phase II wet AMD study, expected to begin this year. If this kind of efficacy is confirmed, integrin peptide therapy may be useful in combination with anti-VEGF therapy early in the treatment course, then as quarterly-injection monotherapy for longer-term maintenance.
Diabetic macular edema
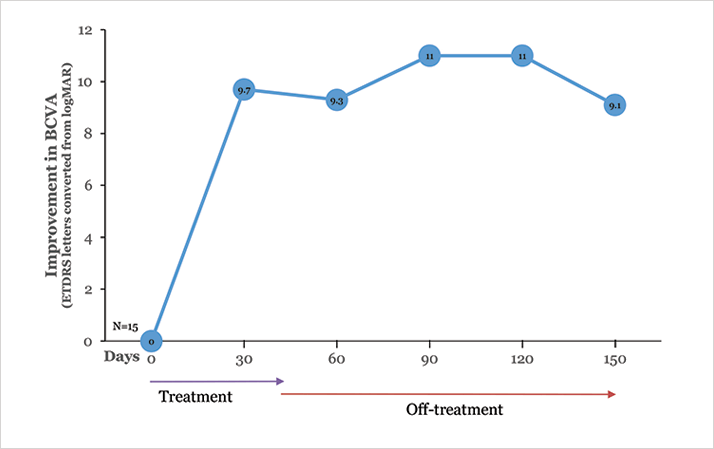
Diabetic retinopathy is a complex disease in which a number of pathogenic pathways are implicated. Despite the success of ranibizumab in the RIDE/RISE trials and aflibercept in the VIVID/VISTA-DME trials, it is reasonable to expect that a combination of therapies will ultimately prove to be most effective against this multifactorial disease.Fifteen subjects with DME were enrolled in an initial safety (phase I) trial for ALG-1001. These were not straightforward, naïve-to-treatment cases. Rather, many were subjects with end-stage DME refractory to repeated anti-VEGF treatment. After a 90-day washout period, the 2.0 mg dose was given monthly by intravitreal injection for three months, followed by three months of (off-treatment) follow up. The safety profile was excellent, with no patients losing vision and no serious adverse events. Slightly more than half the patients improved to functional visual acuity (20/40 to 20/60) in this monotherapy trial (Figure 3). As in the AMD study, there was a corresponding improvement in central macular thickness and the improvements were maintained for the duration of the study – which in this case included a three-month off-treatment period. A phase Ib/IIa DME trial in which subjects are treated with a combination of ALG-1001 and anti-VEGF agents in comparison to anti-VEGF alone is underway.
Vitreomacular traction
An additional endpoint in the phase I DME study was posterior vitreous detachment (PVD), observed by video B-scan. Six of the 11 patients in that study who had no or partial PVD at baseline developed total PVD by day 90 (Figure 4). This is very encouraging and not altogether surprising, given that ALG-1001 is known to inhibit the integrin α3β1 receptors associated with the attachment of the vitreous extracellular matrix to the retina.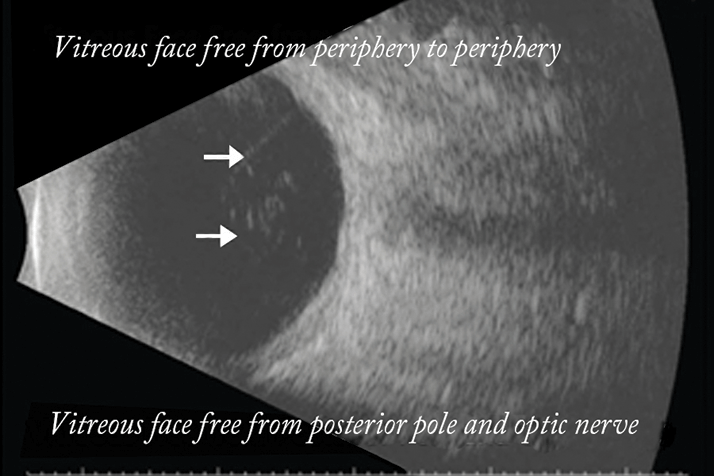
Currently, ocriplasmin is available for the treatment of symptomatic vitreomacular adhesion but it does not usually create a full PVD, particularly in cases with larger areas of adhesion. It would be a boon to the retinal community if the success rate of medical therapy could be increased without any impact on safety; this could help more patients avoid the risk of surgical vitrectomy. Investigators are currently enrolling 60 subjects in a phase II, double-masked, randomized, placebo-controlled study to evaluate the efficacy of ALG-1001 in resolving VMT. If all goes well, the company hopes to move quickly into phase III trials, as ALG-1001 for VMT may fill a significant unmet need and offer the shortest regulatory path of all the indications sought for this drug. All of these trials are still in the early stages, but integrin peptide therapy has progressed from initial molecule discovery to phase II clinical trials rapidly and with encouraging results to date. If we continue to see good efficacy and duration of effect in the larger phase II and phase III studies, particularly with a dose-escalating response, I feel confident that ALG-1001 will have a significant place in our treatment armamentarium. Dr. Boyer is co-founder of the Retina Vitreous Associates Medical Group, in Los Angeles, California and is a leading investigator for various national clinical trials on retinal diseases and an advisor for multiple research, educational and charitable institutions. Dr. Boyer is a member of Allegro’s Scientific Advisory Board and is an equity owner in Allegro. He also serves as a consultant to Genentech and Regeneron.
