Glaucoma is a leading cause of irreversible vision loss, affecting 70 million people globally. This chronic, progressive disease is characterized by elevated IOP – a major risk factor for glaucomatous visual field loss. While eye drop medications are the standard first-line treatment to lower IOP, adherence is an important factor and for some patients this may be challenging (2).
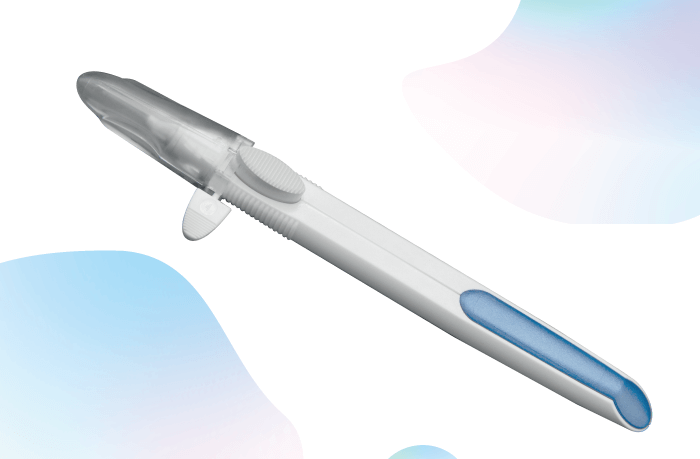
DURYSTA™ (bimatoprost intracameral implant) is the first and only FDA-approved biodegradable, intracameral implant indicated for the reduction of IOP in patients with OAG or OHT. DURYSTA™ comprises a sterile implant containing 10 mcg of prostaglandin analog preloaded into a single-use applicator that should only be administered once per eye without re-treatment. The applicator contains prostaglandin analog, bimatoprost, which is believed to lower IOP in humans by increasing aqueous humor outflow through both the trabecular meshwork and uveoscleral outflow pathways. DURYSTA™ is administered directly into the anterior chamber of the eye, via a pre-loaded 28-gauge needle, where it delivers bimatoprost 24/7 for several months. The implant – measuring approximately 1 mm in length – is made of a preservative-free polymer matrix, designed to slowly degrade over time into lactic and glycolic acid. The benefit for physicians? DURYSTA™ is physician administered, bypassing the ocular surface for direct delivery to diseased tissues (1).
The availability of DURYSTA™ marks a major milestone for IOP lowering in glaucoma.
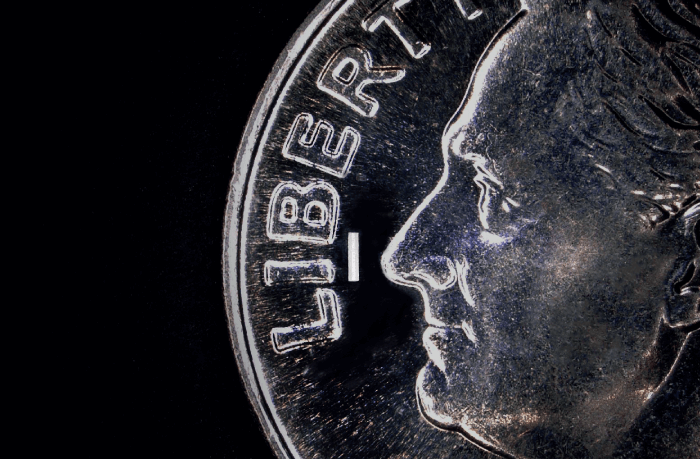
For full Prescribing Information for DURYSTA™, please click here.
For more information, visit durystahcp.com.
DUR142299 11/20
© 2020 Allergan. All rights reserved. All trademarks are the property of their respective owners.
DURYSTA™ (bimatoprost implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
IMPORTANT SAFETY INFORMATION
Contraindications
DURYSTA™ is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade < 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5%-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
References
- DURYSTA™ [Prescribing Information]. Irvine, CA: Allergan, Inc. (2020).
- B Gomes et al., “Assessment of eye drop instillation technique in glaucoma patients”, Arq Bras Oftalmol, 80, 238 (2017). PMID: 28954024.
