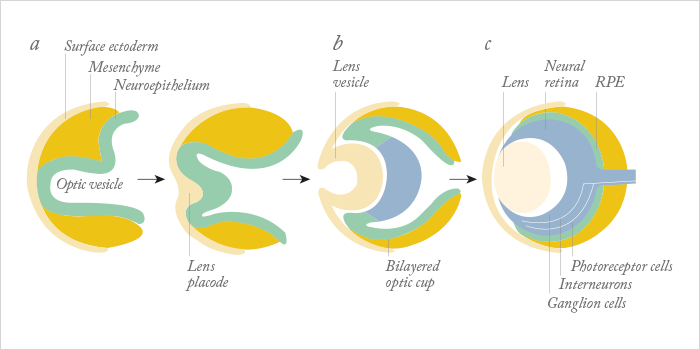
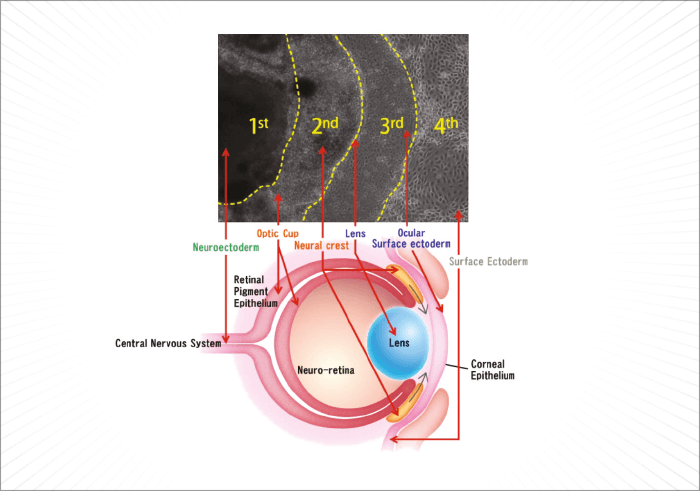
For a human embryo, making an eye is a complex process. It begins during the fourth week of development, is driven by the master control gene, Pax6, requires cells that derive from two of the three major embryonic lineages (Figure 1), and requires a multitude of genes, many of which are transcription factors, to interact in increasingly complex ways to build the structures that will form a functioning eye. Understandably, it’s much harder to do that in vitro – but researchers have managed to develop retina-like structures, which contain cells that express markers of both the neural retina and the retinal pigment epithelium (RPE), by combining multiple embryonic stem (ES) or induced pluripotent stem (iPS) cell sources, with gel scaffolds (1–5). The problem is, those successes haven’t been replicated with ocular surface cells. Until now… In a recent paper published by Hayashi and coworkers in Nature, researchers took a different approach: using human iPS cells to create what they call a “self-formed ectodermal autonomous multi-zone” (SEAM) of ocular cells (6). SEAM mimics the development of the eye, and each zone has cells of different embryonic lineage – ocular surface ectoderm, lens, neuro-retina, and retinal pigment epithelium – and represents a particularly promising approach for the study of ocular morphogenesis (Figure 2). However, the researchers have gone one better: using this SEAM approach, they’ve managed to take cells from the ocular surface (ectodermal zone), sort and expand them in vitro, and grow corneal epithelium sheets that, when transplanted into rabbits with experimentally-induced total epithelial limbal stem-cell deficiency, the transplanted epithelial cell sheet restored a heathy corneal barrier function, and continued to express cornea-specific proteins. The authors claim that they are “now in [a] position to initiate first-in-human clinical trials of anterior eye transplantation to restore visual function”.
In the accompanying Nature editorial (7), Julie Daniels, a professor of regenerative medicine and cellular therapy at the University College London Institute of Ophthalmology, emphasized what this research means for patients and eye research:
- Making corneal epithelial sheets using their stem cell-based method would be prohibitively expensive under current good manufacturing practices
- The SEAM model should speed the discovery of the fundamental mechanisms that underlie the development of each cell type, and may reveal molecules that promote the proliferation of each ocular cell type in ageing adults
- A better understanding of this might eventually enable manipulation of stem cells within the eyes – much like the lens regeneration work reported by Lin et al. (8).
Although corneal transplants and stem cell therapies like Holoclar are successfully used to treat a range of corneal pathologies, today, there are still many cases where the only option is a keratoprosthesis. Hayashi et al.’s work may one day herald a change to that statement.
References
- M Eiraku et al., “Self-organizing optic-cup morphogenesis in three-dimensional culture”, Nature 472, 51–56 (2011). PMID: 21475194. ME Zuber et al., “Specification of the vertebrate eye by a network of eye field transcription factors”, Development 130, 5155–5167 (2003). PMID: 12944429. DA Lamba et al, “Efficient generation of retinal progenitor cells from human embryonic stem cells”, Proc Natl Acad Sci USA, 103, 12769–12774 (2006). PMID: 16908856. F Osakada et al., “Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells”, Nature Biotechnol. 26, 215–224 (2008). PMID: 18246062. C Yang et al., “Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions”, FASEB J. 24, 3274–3283 (2010). PMID: 20410439. R Hayashi et al., “Co-ordinated ocular development from human iPS cells and recovery of corneal function”, Nature, 17, 376–380 (2016). PMID: 26958835. JT Daniels, “Biomedicine: Visionary stem-cell therapies” Nature, 531, 376–380 (2016).PMID: 26958834. H Lin et al., “Lens regeneration using endogenous stem cells with gain of visual function”, Nature, 17, 323–328 (2016). PMID: 26958831.
