In cataract surgery today, lens epithelial cells (LECs) are the enemy. You prep the patient with anesthetic, make the side-port incision and inject some viscoelastic, make your second incision and perform the capsulorhexis, followed by phaco, then irrigate/aspirate, place the IOL in the capsular bag, remove the viscoelastic, and you’re done. And hopefully the patient’s vision improves. That is, unless LECs get in the way and bring on posterior capsule opacification (PCO). The mechanism by which PCO occurs is well-characterized: LECs that remain within the capsule can migrate to the posterior capsule where they undergo aberrant differentiation into fiber-like cells (or transdifferentiation into fibroblast-like cells), where they obscure the central visual axis, causing that hazy vision that patients complain about. A lot of effort goes into preventing this: square-edged IOL design, anterior capsule polishing during the procedure… but what if we’ve got it all wrong? That the LECs aren’t the problem, but it’s the way the cataract surgery is actually performed? Recent research published in Nature makes very interesting reading. LECs are fascinating. They self-renew, cover the lens’ anterior surface, and begin to differentiate into lens fibers at the equator – and appear to have protective capabilities against oxidative damage and external injury. A team led by Lin (1) noticed that LEC proliferation rates decrease with age, but when you remove the entirety of the lens from the capsular bag, LECs proliferate like crazy. Using some elegant immunohistochemical and transgenic mouse experiments, the researchers discovered that Pax6 and Bmi1 – genes that are well-characterized in embryonic eye development and postnatal stem cell maintenance, respectively – play important roles in postnatal lens fiber cell development (Pax6), and maintenance of postnatal stem cell populations (Bmi1). It turns out that the loss of Bmi1 in the eyes of mice disrupts LEC proliferation, thereby promoting cataract formation.
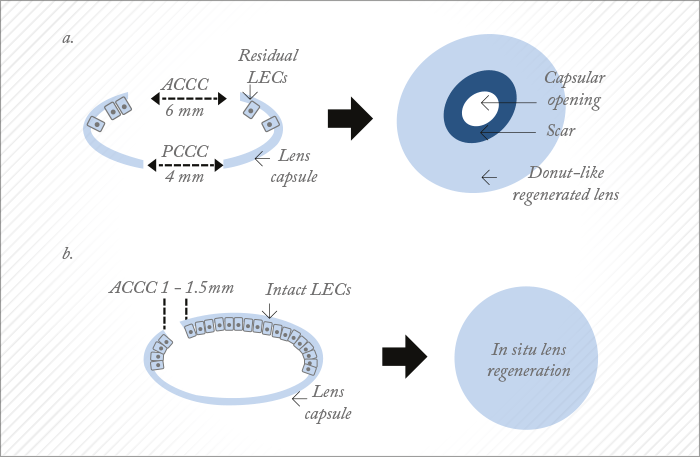
These discoveries alone are valuable additions to the scientific literature, and hint at a role for LECs in in situ lens regeneration. But Lin et al. went on to describe – in the same paper – a way of actually regenerating the lens: a completely new take on capsulorhexis. A typical capsulorhexis involves making an opening right in the center of the anterior capsule – and this results not only in a large wound area, but the destruction of a large number of LECs (Figure 1a). In the paper, Lin and coworkers proposed a minimally invasive alternative, by placing a much smaller ‘rhexis outside of the central visual axis (Figure 1b). The approach was first evaluated in rabbits, with the small capsulorhexis being swiftly followed by careful hydrodissection of the lens cortex from the anterior capsule. This was then removed via irrigation and aspiration from a phacoemulsification device (although no phaco energy was used), and the limbus wound was closed with a single suture. Seven weeks later, the rabbits had regrown a transparent lens that was “comparable to a normal, healthy lens” – including its refractive power. The experiment was repeated in infant macaques, aged 1–3 months – with the same result. And the obvious question: it works in animals, but can it work in humans? Apparently, yes. The eyes of infant macaques are similar to those of human infants aged 4–12 months. If children are born with congenital cataract, they require the removal of their cloudy lenses, and as with adults, a capsulorhexis in the center of the visual axis is made, and the cataractous lens removed. Children aged 2 years and younger don’t receive an IOL and are left aphakic until at least 2 years of age. Even so, there are still numerous complications associated with this procedure, including visual axis opacification, secondary glaucoma, surgery-related complications, and other challenges that can lead to poor visual outcomes. If Lin et al.’s technique worked in infants, it would have profoundly positive consequences for the treatment of congenital cataract.
It does. When the team compared their minimally invasive technique on 12 infants (aged 0–24 months; 24 eyes) with standard-of-care lens removal (25 infants; 50 eyes), they were able to see the lens regenerate in the first group, post-operatively with a slit lamp. The capsular openings healed within one month of surgery; regenerated biconvex lenses had formed by three months post-procedure, and by eight months, the regrown lens was described as being “comparable to a native lens” – with an accommodative response of 2.5 D (significantly greater [p<0.001] than 0.1 D achieved in aphakic controls) that resulted in greatly improved visual acuity relative to baseline, pre-surgery levels (p<0.001). Furthermore, minimally invasive cataract surgery was associated with significantly fewer complications compared with standard-of-care surgery (17 vs. 92 percent) – all because the procedure spared the children’s own stem cells. One of the study’s leaders, Kang Zhang, a professor of ophthalmology and chief of Ophthalmic Genetics at UCSD, said, “We believe that our new approach will result in a paradigm shift in cataract surgery and may offer patients a safer and better treatment option in the future.”
Bmi1 is a polycomb ring finger oncogene. It regulates p16 and p19, two genes that inhibit the cell cycle. The BMI1 protein appears to play a role in DNA repair, but its other key role is that it’s necessary for efficient self-renewing cell divisions of adult hematopoietic, peripheral and central nervous system stem cells. It’s also thought to be an anti-aging gene in neurons, as it acts to suppress the activity of p53.
Pax6 was first identified from patients with aniridia – on investigation, patients had significant deletions to the gene that codes for it. It’s a transcription factor, and in embryonic development, it’s responsible for the formation and maturation of eyes and other sensory organs, certain neural and epidermal tissues. Other Pax6 mutations can cause (in humans) keratitis, optic nerve keratitis and coloboma, morning glory disc anomaly, and cataract with late-onset corneal dystrophy. Pax6’s big claim to fame comes from fruit fly experiments: Drosophila lacking the Pax6 gene develop no eyes, but ectopic expression of Pax6 protein leads to ectopic eye development.
But will it? What of older patients with senile cataract – the ones who undergo the vast majority of the ~22 million cataract surgery procedures each year? As mentioned above, LECs proliferate at an increased rate following injury, so it is possible that this approach could work in older patients too, although it’s likely that it would be at a pace that’s considerably slower than in infants (although the identification of both Pax6 and Bmi1 as drivers of LEC renewal might one day open some doors to speeding the process). But there are also a number of additional problems that would need to be addressed: removing hard cataracts commonly requires the use of phaco energy – something that can damage LECs. This begs the question: is there a role for femtosecond lasers here? Florian Kretz thinks so. “A femtosecond laser would be able to not only make the eccentric ‘rhexis, but also (given the right optics and patient interface) might show great value in pre-fragmenting and softening the nucleus, thereby making the procedure far easier to perform in elderly patients”.
Nevertheless, the aged capsule is almost certainly going to be thicker and less elastic in elderly patients than infants, and this may pose a challenge for an adult lens regeneration approach. Accommodation would still be compromised, and with it, any promise of a patient being “spectacle-free” after the procedure. It’s also an interesting after-care scenario: even assuming that the procedure works as well in people aged 70 as it does in infants aged 7 months, you still have a situation where elderly patients are effectively aphakic for a not insubstantial period of time. Their refraction and visual acuity will continuously change – meaning that their spectacle lens prescription will have to change regularly to keep up. This is in stark contrast to most standard cataract procedures, where almost all patients experience an extremely rapid improvement in vision. Finally, this isn’t a panacea for all cataract procedures – Kretz notes that it’s unlikely to help with posterior polar cataracts, and subcapsular cataracts might prove a pathology too far for this new approach to address, and if there’s a genetic cause for these cataracts, the regrown lens may still opacify. Despite all of that, this is undoubtedly a stunning piece of research, and already a great advance in pediatric ophthalmology – and something that in children at least, requires no new devices (or regulatory approvals). The procedure is now being performed on older pairs of eyes: the world (and a multi-billion dollar industry) awaits the results.
References
- H Lin et al., “Lens regeneration using endogenous stem cells with gain of visual function”, Nature, 531, 323–328 (2016).PMID: 26958831.
