I took an unusual route into ophthalmology. After training in mathematics, I worked on artificial intelligence (AI) optimization before moving into medical devices. The transition was kicked off by a conversation with the Optos Chief Technical Officer David Cairns; their product development programs needed the kind of mathematical expertise I could offer. And, in fact, I was predisposed to work in ophthalmology because, as a young person, I’d seen my grandmother go blind due to diabetic complications. The speed of her sight loss was shocking, and the experience never left me – and so it became a powerful motivator in my efforts to improve the management of diabetic retinopathy (DR). I joined a small prototyping team at Optos, and stayed there for several years working on some incredibly advanced devices. I learnt a lot – Optos is a bit like a University – but, after a while, I began to wonder about the company business model. True, Optos was making the best devices on the market – but as a major PLC, its core aim was to enhance shareholder value, so it focused on the territories that returned the most revenues. Commercially, that makes perfect sense, but I couldn’t help asking myself: what about the other 194 territories on Earth? Once I’d posed that question, there was no going back. I left Optos on a Friday, seven years ago, and started Epipole the following Monday. My aim? To build inexpensive ophthalmic imaging devices for neglected populations.
- 1.3 megapixel camera
- Covers 52 degrees in a single shot, with over 100 degree reach
- Operates in amber spectrum (590 nm), which can help differentiate between oxygenated and deoxygenated blood
- No reflex from surface of retina or cornea
- Main application: DR
Life isn’t always a beach
My first job was to track down Bob Henderson, a retired engineer who had invented the key Optos instrumentation. Eventually, I found him in Ko Samui in Thailand – though asking him to return to Dalgety Bay on the Scottish coast felt like I was pushing my luck, he came back. Suddenly, Epipole was a team of two. Having buy-in from one of the finest optical engineers in the world was a great boost for me, but when I showed Bob a sketch of my idea for a low-cost fundus camera, he just looked at it and said: “No.” And that was the start of two guys shutting themselves away in a tiny pitch-black optics room for months on end. It wasn’t easy. We were trying to solve problems which, for all we knew, might have been unsolvable; many ideas that seem great on paper are confounded when they meet biological tissue. Perhaps the hardest thing about building an ophthalmoscope is that the illumination and the optical axis are on top of each other – all the system wants to do is send reflex back to your sensor! We worked on that problem for about a year and, just as we started to question our sanity, we finally made a series of discoveries that fixed the issue. It was a hugely cathartic moment for us, and opened up the way to device prototyping.Aiming high – and small, and wide
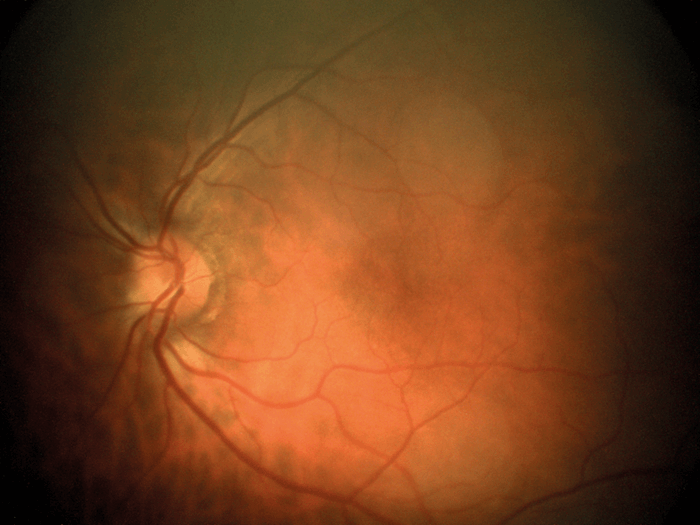
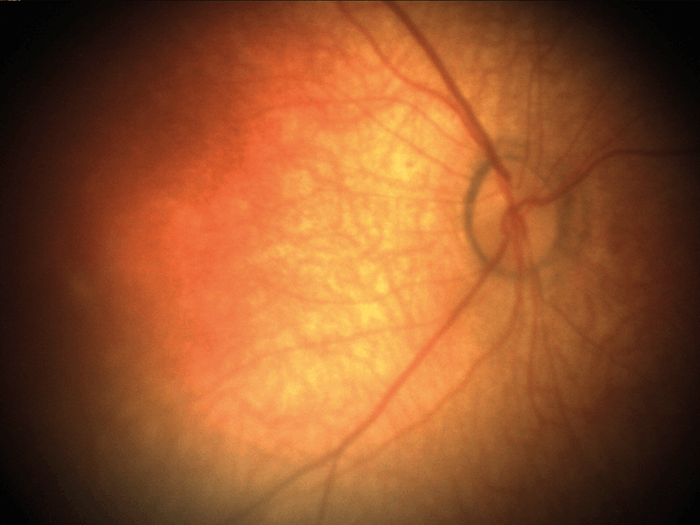
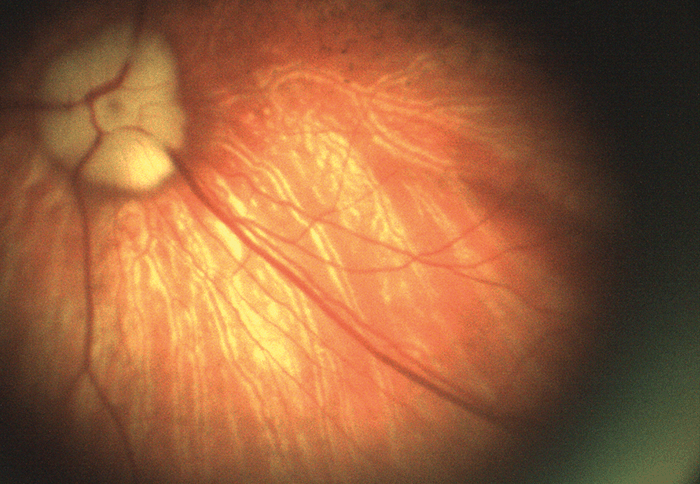
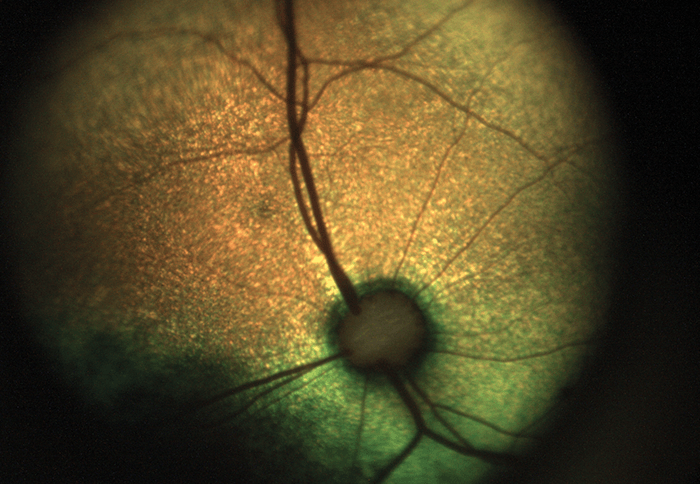
Our first fundus camera, the epiCam M (see Box 1), was conceived to improve the diagnosis of DR. We designed everything about this device from the ground up – the chassis, the optics, the electronics, firmware, software – everything. Nothing similar had been done before, so we were inventing as we went along – and we set ourselves a couple of ridiculously severe engineering constraints. Firstly, we wanted to diagnose DR with a high degree of sensitivity – specifically, by detecting very small microaneurysms (below 10 µm). I didn’t realize how large that hurdle was until we started work! Secondly, we wanted the device to be suitable for any community where DR is an issue, from remote parts of South America to rural China. And that limits size. If you’re taking it to rural communities, it must be portable. It also restricts the price: a rural doctor making $6,000 a year won’t buy a $30,000 camera. The result was better than we could have hoped for: not only did we limit the size and price of the device for our markets, but we also met and exceeded our technical goals. To control camera costs and dimensions, we designed an electronics board (which in itself is something of a work of art!) that permits us to download data via a USB cable and display on a separate screen. Hence, the device does not need a large battery, or a screen, or a computer – the camera body needs only to house the optics. In terms of performance, we achieved a detection limit of about 8 µm at the back of the eye – which is plenty because microaneurysms are only ‘problematic’ when they reach 30–40 µm. We also provided the device with a very wide reach – well over 100 degrees horizontally and vertically, which can help detect pathologies that might be missed by other devices. Indeed, examination of my own eyes with epiCam M identified a congenital hypertrophy of the retinal pigment epithelium (CHRPE) in the periphery, which a market-leading competitor device could not detect. Being able to look around the eye and see pathology beyond 45 degrees, instead of relying on a static view, makes a huge difference. Furthermore, the unique video capability we have incorporated allows observation of the retina in real time as a living tissue. It also supports efficient triage – the doctor can very rapidly examine all around the fundus, which can help make a real difference to clinical practice. The video capability also encourages novel basic science; for example, some are using epiCam M to study blood flow through the eye – you can see vessel dilation and contraction in real time. Others have used it to observe hypoxic changes in the fundus at high altitude. As well as diagnosing DR, the device is also capable of examining related applications, such as the tortuosity of blood vessels, and observing cholesterol platelets. Anecdotally, we have been told by users that it has been useful in a wide range of other systemic conditions.- 5 megapixel camera
- Similar wide reach as epiCam M, but covers 45 degrees per shot
- Operates in full color, red-free and green-free modes
- Like epiCam M, it records video from which still images can be extracted
- Main applications: general ophthalmology, pediatrics
Next generation
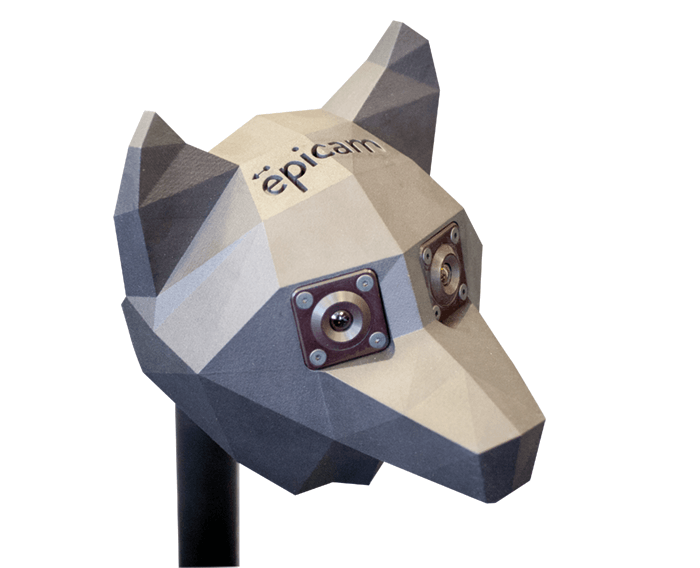
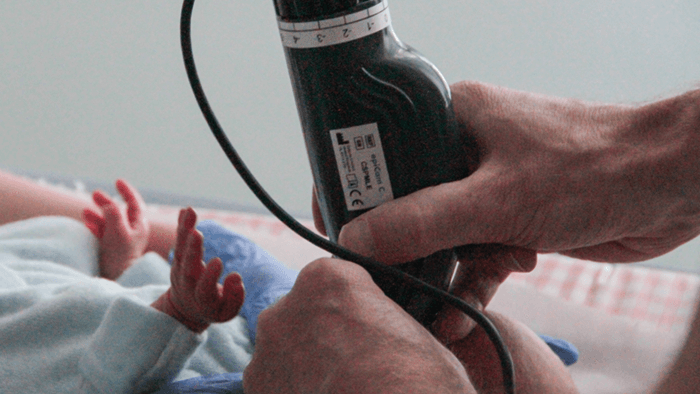
While we were developing the epiCam M, I continued seeking feedback from as many clinicians as I could speak with. And I kept hearing about the need for a better system of diagnosing and monitoring retinopathy of prematurity (ROP). This serious pathology can lead to a lifetime of blindness, and is increasing in frequency with the number of surviving premature neonates. Listening to this feedback, we started developing a second device specifically aimed at ROP: the epiCam C (see Box 2). The device is based on non-contact technology: with a working distance of 13 mm, our device doesn’t touch the infant at all, so an examination is a less stressful experience for all concerned. In fact, clinicians have used our device in the incubator while the child is actually asleep! By contrast, other devices on the market are full-contact and require extensive preparation time. We knew it was important to move away from that strategy. Furthermore, epiCam C is a full-color, video rate ophthalmoscope, making it applicable to general ophthalmology indications as well as pediatrics. We’ve also made a variant of epiCam C – the epiCam V – for veterinary applications (see Box 3), which benefits from low power illumination that can be adjusted to suit the reflectivity of the tapetum – the reflective choroid layer found in most animals. Incidentally, some of the comparative work we see from zoo vets – looking at retinas of Sumatran tigers and Bengal eagle owls and vicunas and monkeys – is just fascinating.Testing times
One of the problems we had to solve when developing our devices was how to test them. Our solution was to develop a proprietary model eye (See Box 4: Model Testing) to assess device performance in terms of resolution and field of view. We now have two versions of this model eye. The first has field rings and a calibration guide (a US Air Force 1951 resolution target), and is designed to test resolving power and performance of ophthalmic equipment – both ours, and that of the competition. The second version is available with a range of printed retinas and serves as a training aid. During its development, we had to invent a way to print onto a hemisphere at 10 times normal resolution, which was a little awkward! We have also combined our model eyes with our model head for a training and demonstration system – something we’ve found to be a massive hit wherever we take it. Increasingly, we get requests for customized models: for example, eyes with various pathologies or bespoke field targets at the back of the eye. One of our clients requested placement of camera sensors at the position of the macula, to exactly simulate what the macula would perceive through the optics of the human eye. And we’ve even launched a dog model head and eye for training veterinary ophthalmologists in the use of epiCam V.- Video capabilities and 5 megapixel camera
- Non-contact imaging
- Low power illuminant that can be adjusted to suit the reflectivity of the tapetum
- 45 degree field of view with reflex-free images


Digesting feedback
Throughout our journey so far, customer feedback has been essential in letting us know we’re on the right track in terms of meeting global clinical needs; for example, revisions to the first generation of epiCam devices were heavily influenced by feedback from Zia Carrim, an ophthalmologist based in Mauritius. The epiCam made a great difference to his clinic, where he sees some very serious cases, but he could still point out areas where we could tweak the device. Similarly, when I was in India in 2013, I had a very useful meeting with Professor Azad at the All India Medical Institute. He was only going to give me five minutes, but when he realized I was interested in what he actually needed, rather than just trying to sell him something, he gave me two hours! Such market research is incredibly valuable – I learnt about his workload, the kind of diseases they see, how they treat them, and what their needs are now and in the future. In the same way, we get fantastic input from the veterinary community, which helps us improve the epiCam V. With any suggestions we get from users immediately going into improving the device, we’ve managed to make the devices better and better over the last five years. It helps being a small company, because we can react very quickly to accelerate product development. In fact – and this may upset the engineers – I am willing to say that I think the epiCams are now design-complete.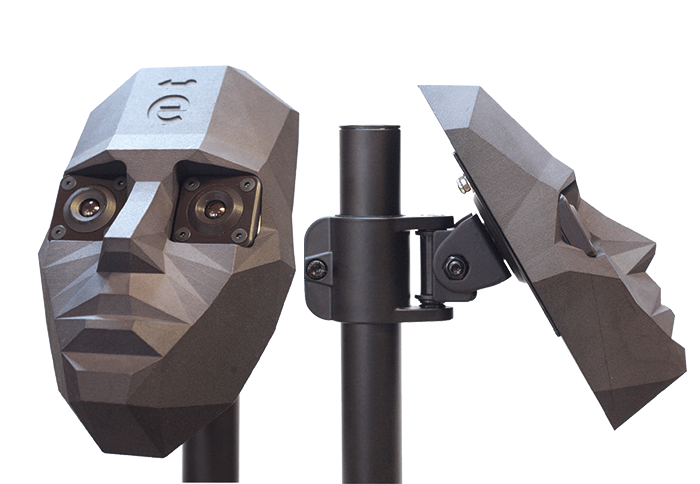
- Corneal shell equivalent to human cornea
- Crystalline lens analogous to lens of human eye
- Axial length identical to that of human eye
- Water-filled; refractive index extremely close to that of vitreous humor
- Customizable to a range of needs: printed retina, different pathologies, sensors at macula
- Fits in model head for convenience and familiarization
- Manufactured by sophisticated 3D-printing – tolerance at the micron scale
- Precise model of human eye for testing or demonstrating any fundus camera or similar device, or for training in the use of such devices
- Users: academia, industry, hospitals