We asked Florent Aptel, Professor of Ophthalmology and Head of the Glaucoma and Anterior Segment Unit, University Hospital of Grenoble, France, for his impressions of PRESERFLO™ MicroShunt.
Where is the biggest need in glaucoma surgery?
Surgeons need a procedure that provides an IOP decrease close to trabeculectomy, but with a much better safety profile and a better predictability. Trabeculectomy is effective, but unpredictable and prone to complications; safer and more reliable alternatives would enable patients to be operated on earlier, to stop eyedrops – thereby reducing incidence of ocular surface disease (OSD) – and increase quality of life. Several studies have demonstrated that roughly 50 percent of patients have been found not to be adherent to their medication over 75 percent of the time (1). Without the necessary treatment, glaucoma can lead to blindness (1) – so we need procedures that more safely and predictably lower IOP.
How does PRESERFLO™ MicroShunt fill the gap?
I find PRESERFLO™ MicroShunt has intrinsic advantages as a filtration device. Firstly, it is made from a highly biocompatible polymer (“SIBS”), which induces less inflammation and less fibroblast proliferation; studies show collagen deposition and myofibroblast differentiation is less with SIBS than with silicone devices (2). This biocompatibility is important when you consider that the main cause of filtration surgery failure is bleb fibrosis, and because SIBS is very inert in biological systems (3), it is stable over time.
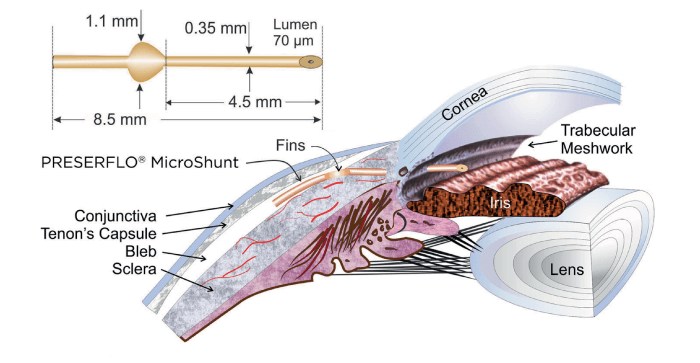
Secondly, PRESERFLO™ MicroShunt’s development pathway included three design modifications and four clinical trials, resulting in a very high qualified success rate in the clinical studies performed to date (4). This iterative process has resulted in a device with ideal dimensions: the 70 μm lumen was sufficient for aqueous humor flow unhindered by blockages related to sloughed cells or pigment while at the same time sufficiently small to prevent hypotony (5). And its 8.5 mm length supports posterior bleb formation, which is more comfortable for patients and offers better long-term outcomes. PRESERFLO™ MicroShunt has paired fins that stabilize the device in the scleral pocket, which helps resist post-surgical displacement and avoid incision-site leakage (and any resulting hypotony).
The fins also assist in the estimation of distance between device and limbus, and the extent of protrusion into the anterior chamber (AC). By modulating intra-AC protrusion, we can avoid contact with corneal endothelium without risking inadequate filtration. The functionality of the design when combined with the absence of inflammation-mediated capsule formation likely explains why PRESERFLO™ MicroShunt results in efficacy close to trabeculectomy that is sustained over two years (6).
How straightforward is PRESERFLO™ MicroShunt procedure?
Ab externo insertion of PRESERFLO™ MicroShunt creates a pathway for aqueous humor to flow from the anterior chamber through the subconjunctival
spaces and into a filtering bleb underthe upper lid. Having been trained in ab externo procedures, such as trabeculectomy and deep sclerectomy, glaucoma surgeons are accustomed to this approach – an advantage over ab interno methods – and most surgeons are comfortable with the device after only 10 to 15 procedures.
Notably, PRESERFLO™ MicroShunt surgery is simpler than deep sclerectomy or trabeculectomy – scleral flaps and Schlemm’s canal dissections are unnecessary. All the necessary tools are provided with the kit; implantation does not need gonioscopy, and application of mitomycin C sponges and washes is simple. Although the use of the antifibrotic agent mitomycin C for this purpose is off label, it is standard practice according to the state of the art for the surgical treatment of glaucoma. The whole procedure requires only eight to 10 minutes of surgeon time and enables surgeons to make larger, more posterior blebs. Topical anesthesia is sufficient, so hospitalization is unnecessary (trabeculectomy patients, by contrast, may need to remain in hospital for 24 to 48 hours). Given the COVID-19 pandemic, this particular advantage is even more important right now.
Other advantages of PRESERFLO™ MicroShunt subconjunctival route over trabecular and uveoscleral pathways include the unhindered aqueous flow that it permits. Trabecular pathway transit, by contrast, is limited by flow resistance in tissues around Schlemm’s canal, while uveoscleral-mediated flux is unpredictable.
Which patients are suitable for PRESERFLO™ MicroShunt?
PRESERFLO™ MicroShunt is suitable for use in patients with primary open angle glaucoma where IOP remains uncontrollable while on maximum tolerated medical therapy and/or where glaucoma progression warrants surgery (7). I have also used PRESERFLO™ MicroShunt both as a standalone procedure and alongside cataract surgery in a combined procedure. Given the low risk of bleb fibrosis, those with thick and inflammatory conjunctiva are also suitable candidates.
Describe your experience with PRESERFLO™ MicroShunt.
I first used it five years ago, as an investigator in a European multicenter clinical trial; I implanted PRESERFLO™ MicroShunt in around 30 POAG patients (18–35 mmHg baseline IOP on maximally tolerated glaucoma medications). I’m still involved in ongoing clinical trials, and have now implanted around 150 PRESERFLO™ MicroShunts.
My preliminary observation in my clinical practice is that around 80 percent of PRESERFLO™ MicroShunt patients retain well-controlled IOP over two years or more, without additional surgery or medication. Furthermore, they have different blebs than XEN® (Allergan Ltd) recipients, which is to say, more posteriorly-located, more diffuse, thicker walls, lower vascularity, and lower incidence of symptomatic dysesthesia – no uncomfortable, prominent blebs.
Importantly, PRESERFLO™ MicroShunt gives lower, more predictable IOP than XEN®. Trabeculectomy may offer slightly greater (~2 mmHg) mean IOP reduction, but with higher risk of hypotony. Once again, PRESERFLO™ MicroShunt IOP reduction is more predictable: 80–90 percent of patients have IOP of 8–15 mmHg one or two years post-surgery. This reduction applies even where preoperative IOP is very high (>30 mmHg). Furthermore, PRESERFLO™ MicroShunt allows us to cease glaucoma medications in 70–80 percent of patients within two years, and reduce them in the rest. And that means fewer side effects, reduced OSD, and better quality of life.
Post-operative management is minimal: bleb needling or bleb revisions are rarely necessary (~10 percent needling/revision rate over 2 to 4 years, comprising ~6.5 percent needling and ~3.5 percent revisions). In contrast, with XEN® needling rate is up to 40 percent, and with trabeculectomy we often have to see the patient again quite soon after surgery – for example, to re-suture the conjunctival incision because of leakage or to fix bleb fibrosis. Uncomplicated post-operative management is good for everyone – surgeons, community ophthalmologists, and patients, and helps reduce virus transmission in times of COVID-19.
PRESERFLO™ MicroShunt has a favorable safety profile. We have not seen hypotony, macular or papillary edema cataracts, decreased visual acuity or bleb leaks although these adverse events were listed in the IFU (7); recovery of visual function is much more rapid with PRESERFLO™ MicroShunt than with trabeculectomy.
Finally, what do patients think of the procedure?
The fact that we get no complaints from PRESERFLO™ MicroShunt patients is very telling! They are happy to have a rapid procedure (with fast recovery and minimal discomfort) that enables them to cease medication. Surgeons are happy to use PRESERFLO™ MicroShunt for similar reasons. For me, the device meets a significant need in glaucoma management.
The publication of this interview was supported by Santen.
References
- A Robin, DS Grover, “Compliance and adherence in glaucoma management,” Indian J Ophthalmol, 59, S93 (2011). PMID: 21150041.
- A Acosta et al., “A newly designed glaucoma drainage implant made of poly(styrene-bisobutylene-b-styrene),” Arch Ophthalmol, 124, 1742 (2006). PMID: 17159034.
- L Pinchuk et al., “Medical applications of poly(styrene-b-isobutylene-b-styrene) (SIBS),” Biomaterials, 29, 448 (2008). PMID: 17980425.
- L Pinchuk et al., “The development of a micro-shunt made from poly(styrene-bisobutylene-b-styrene) to treat glaucoma,” J Biomed Mater Res Part B, 105B, 211 (2017). PMID: 26380916.
- O Sadruddin et al., “Ab externo implantation of the MicroShunt, a poly(styrene-b-isobutylene-bstyrene) surgical device for the treatment of primary open-angle glaucoma: a review,” Eye and Vision, 6, 36 (2019). PMID: 31807606.
- L Pinchuk et al., “The use of poly(styrene-bisobutylene-b-styrene) as a microshunt to treat glaucoma,” Regenerative Biomaterials, 2016, 137 (2016). PMID: 27047682.
- PRESERFLO™ MicroShunt glaucoma drainage system. Instructions for Use
